Projects & Partners
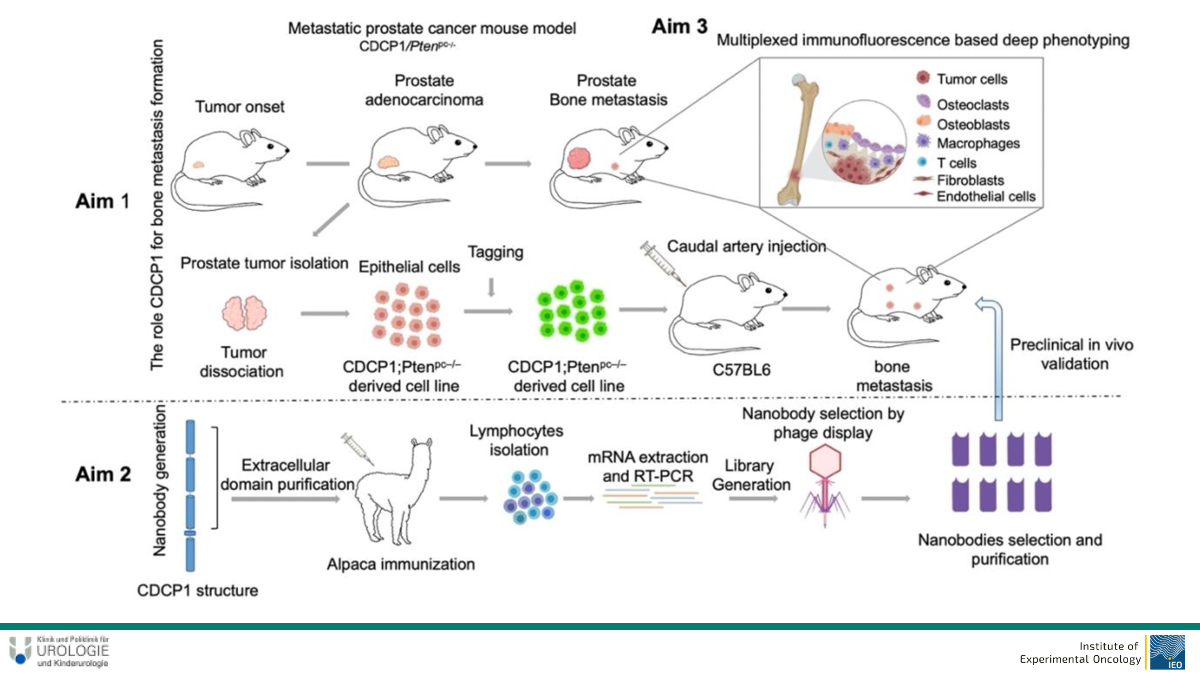
Deciphering the role of CDCP1 for priming the bone metastatic niche in prostate cancer XX




→ Mouse models
→ Tumor cell lines
→ High-plex immunofluorescence (CODEX)
→ Genome engineering
→ Cancer Immunotherapy
Prostate cancer (PCa) is one of the top leading causes of cancer related deaths among men in developed countries. Approximately 10% of newly diagnosed PCa patients present with bone metastais, increasing to 80% at advanced stages of the disease. Bone metastases are associated with poor prognosis, bone pain , skeletal fractures and indicate the incurability of disease in most cases. The bone metastatic niche is a complex microenvironment made of osteoblasts, osteoclasts, stromal cells, extracellular matrix, vessels and immune cells, which together with tumor cells comprise and shape the metastatic niche. Cub domain containing protein 1 (CDCP1) is a transmembrane protein that acts as a substrate for Src tyrosine kinase family. CDCP1 is overexpressed in various cancer types and associated with poor prognosis and metastasis. It was shown to cooperate with HER2 in breast cancer to promote activation of Src, while CDCP1 protein levels were elevated in metastatic prostate cancer, and specifically in castration-resistant prostate cancer (mCRPC) patients.
In this project, we aim to decipher the role of CDCP1 in bone metastsis formation, and its spatial, phenotypic and functional interaction with the other compartments of the bone metastatic microenvironment. To do that, we will use a metastatic Pca mouse model that allows the transgenic overexpression of CDCP1 in parallel with the loss of Pten expression, in prostate tissue, and develops highly aggressive carcinoma. State of the art techniques, such as multi-color flow cytometry in combination with ultra high multiplexed immunofluorescence (CODEX) will be performed for deep phenotypic and spatial characterization of the bone niche. Moreover, to address the functional contribution of CDCP1 to bone metastasis, we will generate mouse PCa cell lines with either high or low activity of CDCP1, using genome engineering approaches.
Lastly, we will develop a therapeutic approach to block CDCP1 signaling, to target bone metastasis development and progression. Syngeneic mice injected with PCa cells will be treated with CDCP1 specifc nanobodies, and bone metastasis formation will be monitored.
Overall, our project will sead light in the interactions of bone compartments during prostate metastasis, will reveal the role of CDCP1 in these intratumoral interactions, and will attempt to generate novel therapeutic strategies to prevent or regress bone metastasis.
- Alajati, Abdullah, Mariantonietta D’Ambrosio, Martina Troiani, Simone Mosole, Laura Pellegrini, Jingjing Chen, Ajinkya Revandkar, et al. 2020. “CDCP1 Overexpression Drives Prostate Cancer Progression and Can Be Targeted in Vivo.” The Journal of Clinical Investigation 130 (5): 2435–50.
- Alajati, Abdullah, Ilaria Guccini, Sandra Pinton, Ramon Garcia-Escudero, Tiziano Bernasocchi, Manuela Sarti, Erica Montani, et al. 2015. “Interaction of CDCP1 with HER2 Enhances HER2-Driven Tumorigenesis and Promotes Trastuzumab Resistance in Breast Cancer.” Cell Reports 11 (4): 564–76.
- Chen, Jingjing, Ilaria Guccini, Diletta Di Mitri, Daniela Brina, Ajinkya Revandkar, Manuela Sarti, Emiliano Pasquini, et al. 2018. “Compartmentalized Activities of the Pyruvate Dehydrogenase Complex Sustain Lipogenesis in Prostate Cancer.” Nature Genetics 50 (2): 219–28.
- Effern, Maike, Nicole Glodde, Matthias Braun, Jana Liebing, Helena N. Boll, Michelle Yong, Emma Bawden, et al. 2020. “Adoptive T Cell Therapy Targeting Different Gene Products Reveals Diverse and Context-Dependent Immune Evasion in Melanoma.” Immunity 53 (3): 564–80.e9.
- Glodde, Nicole, Tobias Bald, Debby van den Boorn-Konijnenberg, Kyohei Nakamura, Jake S. O’Donnell, Sabrina Szczepanski, Maria Brandes, et al. 2017. “Reactive Neutrophil Responses Dependent on the Receptor Tyrosine Kinase c-MET Limit Cancer Immunotherapy.” Immunity 47 (4): 789–802.e9.
- Guccini, Ilaria, Ajinkya Revandkar, Mariantonietta D’Ambrosio, Manuel Colucci, Emiliano Pasquini, Simone Mosole, Martina Troiani, et al. 2021. “Senescence Reprogramming by TIMP1 Deficiency Promotes Prostate Cancer Metastasis.” Cancer Cell 39 (1): 68–82.e9.
- Park, Simone L., Anthony Buzzai, Jai Rautela, Jyh Liang Hor, Katharina Hochheiser, Maike Effern, Nathan McBain, et al. 2019. “Tissue-Resident Memory CD8 T Cells Promote Melanoma-Immune Equilibrium in Skin.” Nature 565 (7739): 366–71.
- Reinhardt, Julia, Jennifer Landsberg, Jonathan L. Schmid-Burgk, Bartomeu Bibiloni Ramis, Tobias Bald, Nicole Glodde, Dorys Lopez-Ramos, et al. 2017. “MAPK Signaling and Inflammation Link Melanoma Phenotype Switching to Induction of CD73 during Immunotherapy.” Cancer Research 77 (17): 4697–4709.
- Riesenberg, Stefanie, Angela Groetchen, Robert Siddaway, Tobias Bald, Julia Reinhardt, Denise Smorra, Judith Kohlmeyer, et al. 2015. “MITF and c-Jun Antagonism Interconnects Melanoma Dedifferentiation with pro-Inflammatory Cytokine Responsiveness and Myeloid Cell Recruitment.” Nature Communications 6 (November): 8755.
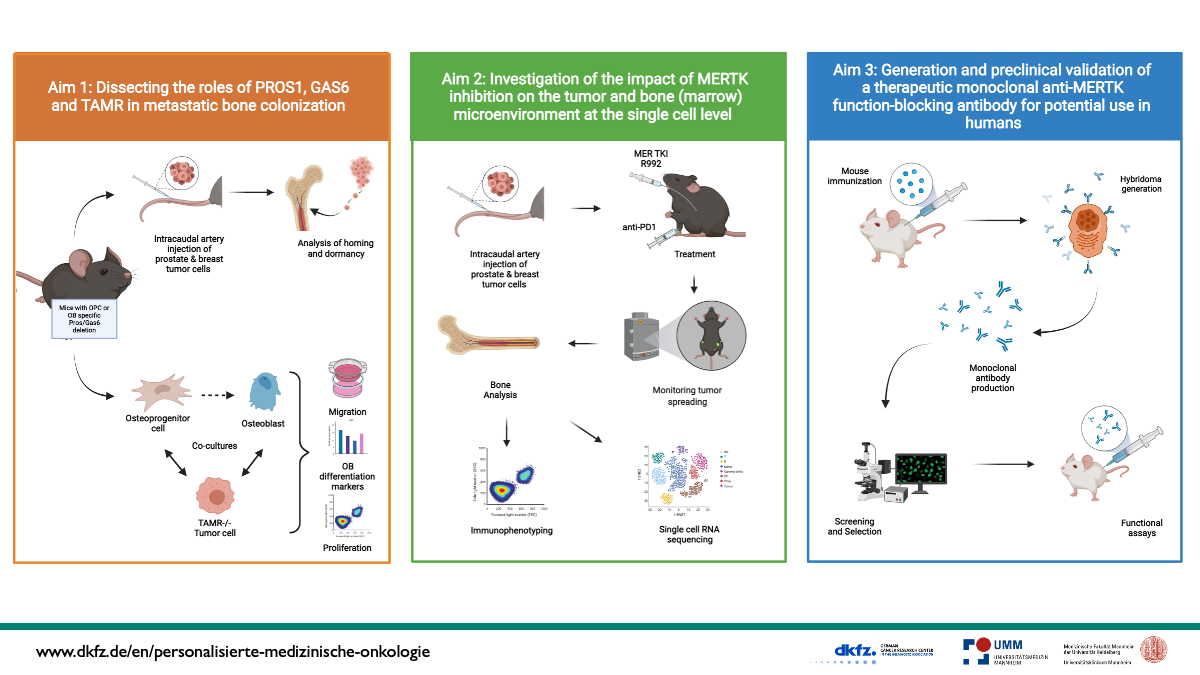
Dissecting the role of TAM receptor-ligand interactions in mediating homing and bone colonization by bone seeking cancers



→ Different murine tumor models (intracaudal injection, orthotopic breast cancer models).
→ In vitro co-cultures of primary cells (osteoblast, osteoclast, NK, T cells) and tumor cell lines.
→ Screening of immune populations (FACS analysis).
The TAM family of receptor tyrosine kinases, consisting of TYRO3, AXL and MERTK and their cognate ligands growth-arrest-specific gene-6 (GAS6) and protein S (PROS1) are aberrantly expressed in a wide variety of cancer entities and promote oncogenesis. It was shown that bone-seeking tumors including multiple myeloma, lung- and breast cancer express GAS6 and PROS1, in addition the GAS6-TYRO3 axis induces osteoclast (OC) differentiation. Consistently, germline deletion of TYRO3 leads to increased bone mass. However, nothing is known about the cell type-specific functions of TAMR in bone cells and the function of PROS1 in cancers metastasizing to the bone.
Therefore, during the first funding period of µBone we investigated the role of PROS1 and TAMR in bone homeostasis and studied the therapeutic potential of the small molecule MERTK inhibitor R992. Our most important discovery is that treatment of bone-seeking myeloma-, breast- and lung cancer mouse models with R992 exerted a potent osteoanabolic effect by counteracting cancer-induced osteopenia and normalizing bone homeostasis. Mechanistically, this was achieved by a direct and specific stimulation of osteoblast (OB) differentiation via blockade of the PROS1-MERTK-RhoA-ROCK axis. Altogether, the targeting of MERTK opens up new avenues for the treatment of bone seeking cancers and their associated morbidities due to osteopenia/osteolysis.
Based on our findings, for the second µBone funding period we aim to dissect the roles of PROS1, GAS6 and TAMR in metastatic bone colonization by breast and prostate cancer. We hypothesize that TAMR ligands, which are highly expressed by osteoprogenitor cells (OPC) and OB, attract tumor cells to the endosteal niche. In addition, we will study the impact of MERTK blockade on the bone microenvironment at the single cell level including effects on important immune cell populations eliciting anti-tumor immune responses in the bone marrow. To approach these questions, we will perform in vitro cocultures and in vivo studies, applying genetic modulation and pharmacological inhibition of MERTK alone or in combination with immunotherapy. An comprehensive number of analysis including live imaging, FACS, histology or RNA sequencing will uncover mechanisms driving cancer cell homing and bone colonization. For potential clinical translation, we will develop and preclinically validate anti-human MERTK function blocking antibodies, for possible human use to treat bone seeking cancers and cancer-induced osteopenia. If successful we will seek for additional funding for a clinical trial after the next funding period of µBone.
Altogether, we will substantially increase the knowledge about the TAMR-guided homing of cancers commonly metastasizing to bone and develop a therapeutic antibody targeting this process.
- Engelmann J…..Ben-Batalla I* and Loges S* (*equal contribution). Regulation of bone homeostasis by MERTK and TYRO3. Nat Comm. Accepted July 2022
- Engelmann J…..Ben-Batalla I* and Loges S* (*equal contribution). Stage dependent regulation of osteoclast differentiation by MERTK and TYRO3. In preparation.
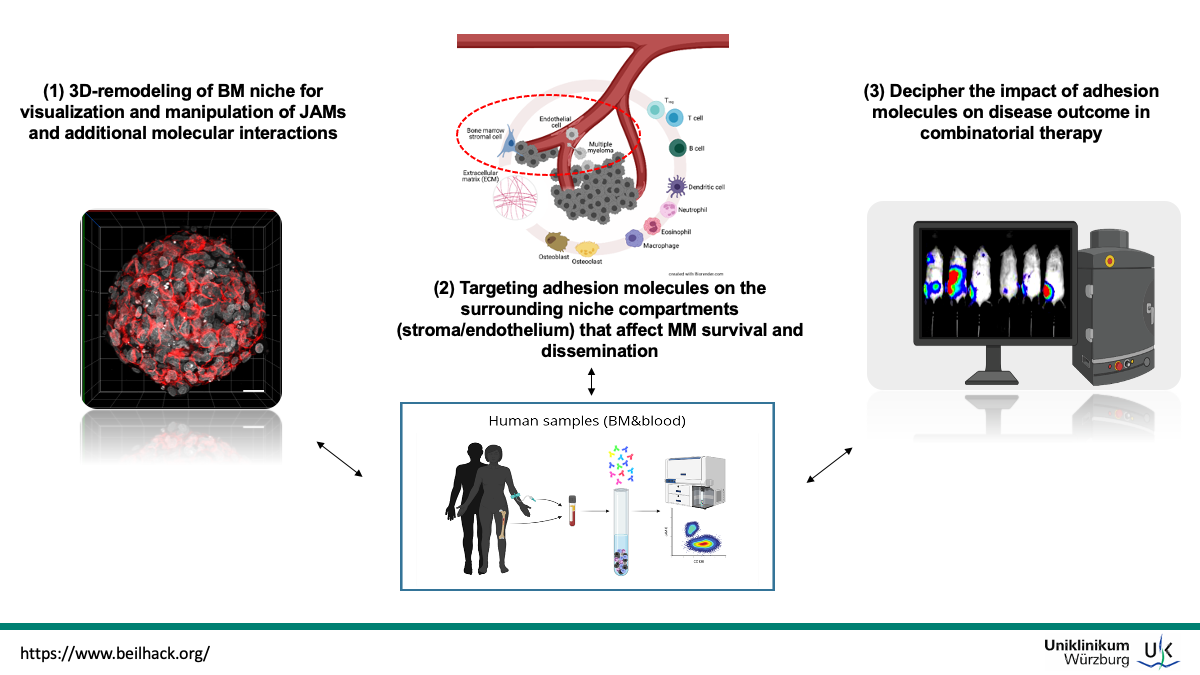
Adhesion molecule interactions at the multiple myeloma interface XX



→ Intratibial injections
→ 3D-Light Sheet Fluorescence Microscopy
→ Myeloma Mouse models and cell lines
Multiple myeloma (MM) is a B-cell malignancy of clonally expanding antibody-producing plasma cells in the bone marrow with a significant impact on morbidity and mortality. Its incidence has steadily increased in the past decades. Though there have been therapeutic advances and major improvements in transplant techniques and new targeted therapies including novel agents (e.g. CAR-T cells, BiTEs, monoclonal antibodies, checkpoint inhibitors etc.) MM remains incurable and has the lowest 5-year survival rate (around 40%) among the three most occurring blood cancers (i.e., lymphoma, leukemia, and myeloma). MM is characterized by dissemination of multiple tumor cells throughout the bone marrow (BM). Disseminating malignant cells will need to migrate through connective tissue and enter the blood circulation using adhesion molecules such as integrins and Ig-like proteins, e.g. members of the Junctional Adhesion Molecule (JAM) family as described for common leukocytes. In recent years it became evident that signaling through adhesion molecules, including the Junctional Adhesion Molecule (JAM)-family members, regulates MM-BM-microenvironment interactions and that their overexpression/dysregulation is implicated in cancer pathogenesis. As the cell adhesion/migration system in the MM microenvironment has been recognized in supporting MM cell survival, progression, and development of drug resistance it became a key target in MM treatment. We postulate that family members of the junctional adhesion molecules (JAMs) play a pivotal role for MM survival, progression, and dissemination by facilitating the crosstalk with cells constituting the BM niche and will address the following specific aims: (I) 3D-remodeling of BM niche for visualization and manipulation of JAMs and additional molecule interactions under conditions which are close to the physiological disease status. (II) Targeting interacting molecules on the surrounding niche compartments (stroma/endothelium) and analyze the effects on survival and dissemination of MM. (III) Decipher the influence of adhesion molecules on disease outcome in combinatorial therapy. To achieve these aims we have established models of orthotopic syngeneic and humanized MM, non-invasive bioluminescence imaging and advanced microscopy techniques (e.g. high-resolution 3D light-sheet fluorescence microscopy). Imaging and microscopy will direct further cellular (e.g. multi-parameter flow cytometry, functional in vitro assays) and molecular in vitro analyses employing sophisticated 3D-co-culture setups based on multicellular micro-tissues. Our studies will help to refine therapeutic interventions, in particularly immunotherapy of MM bone disease. Ultimately, we envision that a better understanding of the pathophysiology of JAMs in MM will result in optimal combinatorial treatment strategies to overcome drug resistance in patients suffering from MM and help to establish semi-personalized anti-MM strategies.
- Brandl et al. “Junctional adhesion molecule C expression specifies a CD138low/neg multiple myeloma cell population in mice and humans.” Blood advancesvol. 6,7 (2022)
- Solimando, AG*; Brandl, A* et al. “JAM-A as a prognostic factor and new therapeutic target in multiple myeloma.” Leukemia vol. 32,3 (2018): 736-743. doi:10.1038/leu.2017.287
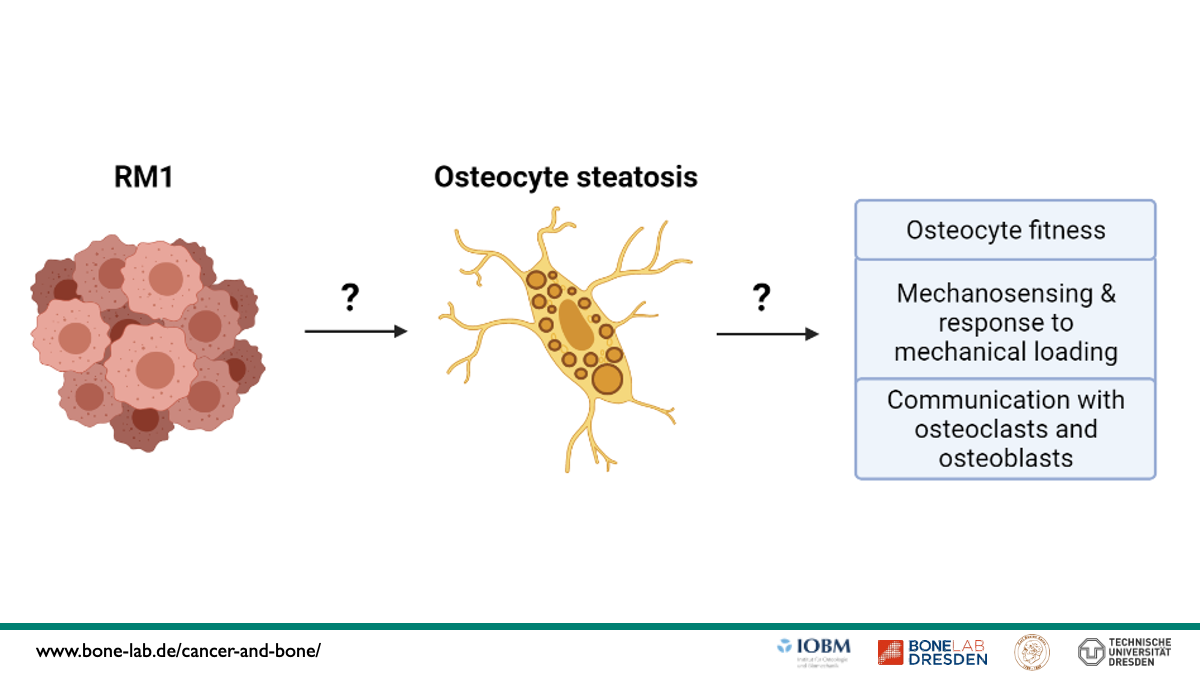
Investigating the role of osteocyte steatosis as underlying mechanisms of the impaired osteocyte network due to bone metastasis




→ High-end imaging
→ Mouse models
→ Tumor cell lines
→ Osteocytes
→ Lipid accumulation
→ Prostate cancer
Osteocytes are the most frequent cell type in bone and are highly interconnected via their lacunar-canalicular network (LCN). Via this communication tool, they orchestrate skeletal adaptation to mechanical stimuli and regulate bone turnover by communicating with osteoblasts and osteoclasts. Recent studies indicate a reciprocal interaction between osteocytes and tumor cells through the bone metastatic process, but little is known about the mechanisms of action and how this affects the response to mechanical loading. In the first funding period, we have identified osteocyte morphological signatures that are specific for osteosclerotic and osteolytic lesions in syngeneic mouse models of breast and prostate cancer bone metastasis models. These alterations, including reduced viability and connectivity, suggest an impaired ability for mechanosensation. Moreover, in vitro, we identified that the osteocyte-like MLO-Y4 cells undergo marked metabolic changes and acquire an adipogenic phenotype characterized by lipid accumulation after conditioning with RM1 prostate cancer cell supernatants. To that end, we have now formulated the hypothesis that due to lipid accumulation in osteocytes, i.e. osteocyte steatosis, mechanosensation via their LCN and communication with bone cells is impaired during bone metastasis conditions. To investigate this further, we defined three specific aims: (I) investigate the mechanism that leads to osteocyte steatosis after treatment with RM1 supernatants and characterize its impact on osteocyte function (i.e. mechanosensing and communication to osteoblasts and osteoclasts) in vitro, (II) visualize lipid droplets and other organelles related to fat metabolism (peroxisomes and mitochondria) in osteocytes and their LCN during prostate cancer bone metastasis and assess the impact of tumor cells on osteocyte response to mechanical loading in vivo, and III) validate key findings in biopsies from bone metastasis from patients with prostate cancer. This project will provide novel insights into the metabolic changes that osteocytes undergo upon tumor cell contact at unprecedented resolution and may deliver new molecular mechanisms that can be harnessed to interfere with the malignant crosstalk between the bone-central regulator osteocytes and tumor cells.
- Wölfel EM, Lademann F, Hemmatian H, Blouin S, Messmer P, Hofbauer LC, Busse B, Rauner M, Jähn-Rickert K, Tsourdi E. Reduced Bone Mass and Increased Osteocyte Tartrate-Resistant Acid Phosphatase (TRAP) Activity, But Not Low Mineralized Matrix Around Osteocyte Lacunae, Are Restored After Recovery From Exogenous Hyperthyroidism in Male Mice. J Bone Miner Res. 2023 Jan;38(1):131-143.
- Hemmatian H, Conrad S, Furesi G, Mletzko K, Krug J, Faila AV, Kuhlmann JD, Rauner M, Busse B, Jähn-Rickert K. Reorganization of the osteocyte lacuno-canalicular network characteristics in tumor sites of an immunocompetent murine model of osteotropic cancers. Bone. 2021 Nov;152:116074.
- Picke AK, Sylow L, Møller LLV, Kjøbsted R, Schmidt FN, Steejn MW, Salbach-Hirsch J, Hofbauer C, Blüher M, Saalbach A, Busse B, Rauner M, Hofbauer LC. Differential effects of high-fat diet and exercise training on bone and energy metabolism. Bone. 2018 Nov;116:120-134.
Dissecting the role of extracellular vesicles and their miRNA cargo in prostate cancer. From expression pattern to bone metastasis phenotype XX


→ Tumor mouse models
→ Prostate cancer cell lines
→ Translational expertise in malignant bone diseases
→ miRNAs
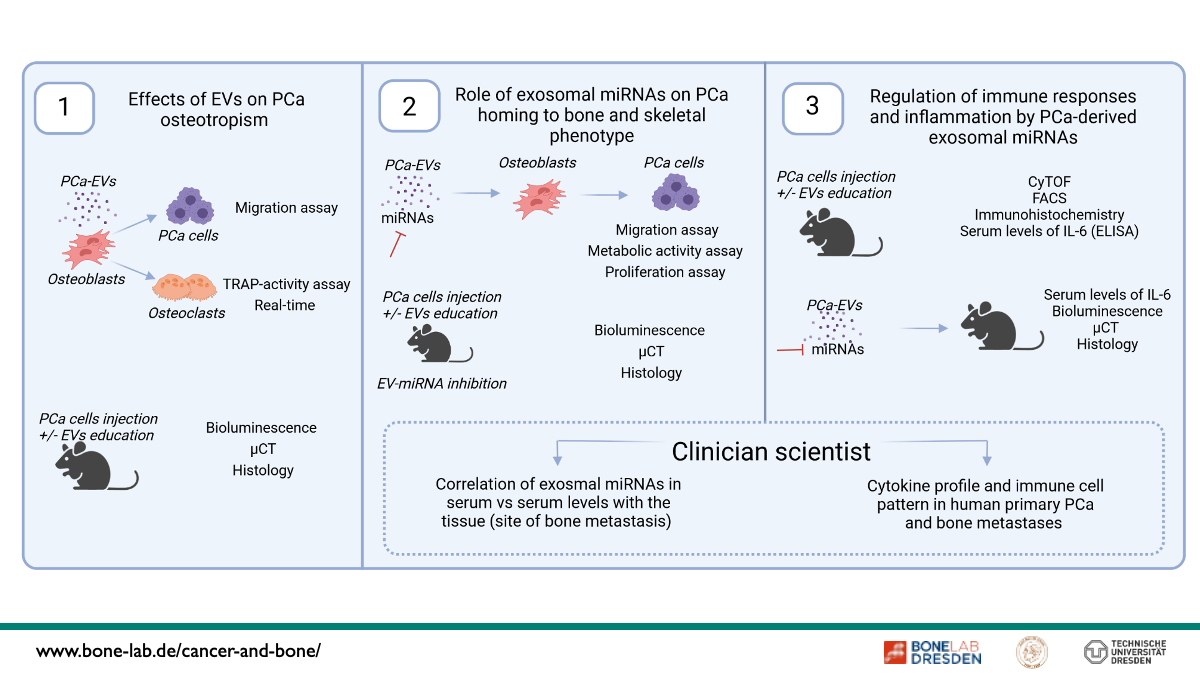
Metastatic colonization of the skeleton is a common complication of advanced prostate cancer (PCa). PCa cells can alter the bone microenvironment even before the metastatic spread, suggesting the presence of a pre-metastatic niche. Extracellular vesicles (EVs) have emerged as crucial players in tumor initiation and progression, contributing to the establishment of a pre-metastatic niche. MicroRNAs (miRNAs) are small non-coding RNAs that may mediate some of the effects of EVs in the cancer-bone dialogue. Our previous studies indicate that PCa-EVs impair osteogenesis and bone mineralization by transferring miRNAs, thereby modifying bone formation, the inflammatory response of osteoblasts, and the phenotype of bone metastases. In this project, we hypothesize that EVs and their miRNA cargo control PCa homing to bone, impair osteoblast and osteoclast functions, and foster immune evasion. We propose that these alterations contribute to PCa progression in bone and regulate the phenotype of bone metastases.
Our specific objectives are (I) to assess the effects of EVs on PCa osteotropism in vitro and in vivo, including tumor migration and associated bone cell cross-talks; (II) to decipher the effects of miR-26a-5p, miR-27a-3p, and miR-30e-5p on PCa homing to bone in murine models and human disease. This includes comprehensive analysis of these miRNAs on migration and other determinants of tumor progression as well as interactions with bone remodeling using murine models and human tissues from our BoneBank that allows a link to the skeletal phenotype (osteosclerostic/ osteolytic); (III) to define osteoimmune mechanisms how EV-derived miRNAs in PCa pre-educate the bone microenvironment. This involves a comprehensive characterization of the bone immune microenvironment in PCa with mass cytometry (CyTOF), followed by confirmation with immunohistochemistry and cytokine assays, focusing on IL-6 signaling. Results will then be validated in human material from the BoneBank. These studies actively involve a clinician-scientist. Elucidation of these processes and mechanisms in preclinical, murine models, and human disease could pave the way for targeted interference with these disease-causing mechanisms.
- Furesi G, Domingues A, Alexopoulou D, Hackl M, Kurth T, Taipaleenmäki H, Conrad S, Rauner M, Hofbauer LC. Exosomal miRNAs from prostate cancer impair osteoblast function in mice. Int J Mol Sci. 2022; 23(3):1285.
- Hemmatian H, Conrad S, Furesi G, Mletzko K, Krug J, Faila AV, Kuhlmann JD, Rauner M, Nusse B, Jähm-Rickert K. Reorganization of the osteocyte lacuni-canalicular network characteristics in tumor sites of an immunocompetent murine model of osteotropic cancers. Bone. 2021, 152:116074.
- Hofbauer LC, Bozec A, Rauner M, Jakob F, Perner S, Pantel K. Novel approaches to target the bone microenvironment in bone metastasis. Nature Rev Clin Oncol 2021; 18(8):488-505.
- Kisel W, Conrad S, Borkowetz A, Furesi G, Füssel S, Sommer U, Rauner M, Thomas C, Baretton GB, Schaser KD, Hofbauer C, Hofbauer LC. High stroma-derived WNT5A is an indicator for low-risk prostate cancer. FEBS Open Bio. 2021; 11:1186-1194.
- Furesi G, Rauner M, Hofbauer LC. Emerging players in prostate cancer-bone niche communication. Trends Cancer 2021;7:112-121.
Molecular characterization and targeting of radioresistant prostate cancer
stem-like cell populations with bone metastasis-initiating properties



PI


→ Tumor cell lines
→ 2D and 3D primary cell cultures
→ CTC analysis
→ Xenograft, syngeneic and GE mice models
→ CRISPR/Cas9 gene-editing
→ Cancer stem cell in vitro and in vivo analyses
→ Radiobiological in vitro and in vivo analyses
→ Retrospective and prospective biomarker analyses
→ Immunohistochemical analyses
→ Mining gene expression datasets
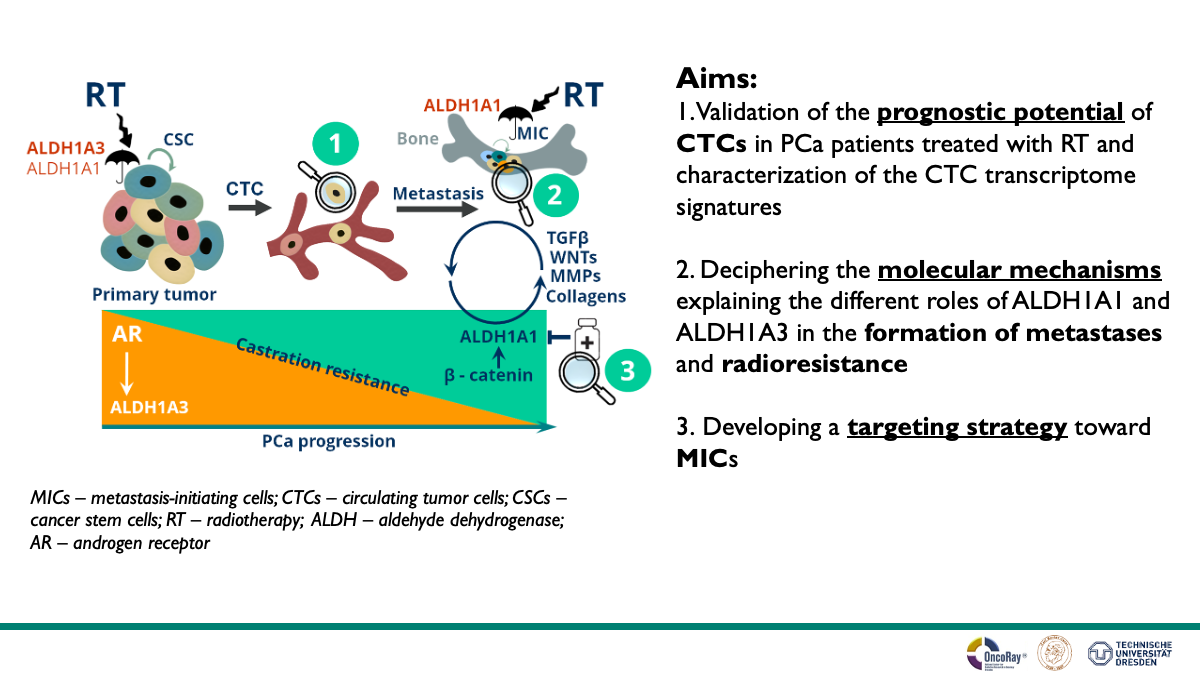
Metastatic tumors cause more than 90% of cancer-related deaths. Prostate cancer (PCa) bone metastases have a heterogeneous response to treatment. Current therapies for osseous metastatic disease, including radiotherapy, frequently fail to provide a durable treatment response. To date, there are only limited therapy options for metastatic PCa, the mechanisms that drive the survival of bone metastasis-initiating cells (MIC) are poorly characterized, and reliable prognostic markers are missing. During the first funding period, we characterized the prognostic potential and the heterogeneity of CTC in metastatic castration-resistant PCa (mCRPC) patients treated with ablative radiotherapy to the metastatic lesions (n = 100). We have employed different in vivo models of metastatic PCa, such as xenograft and syngeneic mice models, and xenograft zebrafish models to demonstrate that prostate tumor cell survival in blood circulation, tumor homing to bone and bone marrow colonization is positively regulated by ALDH1A1 and negatively regulated by ALDH1A3 proteins. Furthermore, immunohistochemical analysis of ALDH1A1 expression levels showed its significant upregulation in distant metastases compared to the primary tumor sites. This analysis also showed that the expression level of ALDH1A1 in primary tumors negatively correlates with disease-free survival in PCa patients. We found that β-catenin triggered ALDH1A1 transcription positively regulates cell radioresistance, tumor cell survival in the blood vessels, and extravasation. Our study showed that the opposite role of ALDH1A1 and ALDH1A3 proteins in tumor cell survival, dissemination, and bone colonization could be partially attributed to their different TGFβ and MPP gene expression regulation. We found that the β-catenin/ALDH1A1 axis is upregulated in radioresistant MIC populations and activates other genes essential for metastatic dissemination and bone colonization. To further explore the molecular characteristics and potential targeting options for radioresistant metastasis-initiating PCa cells, we plan (i) to validate the prognostic potential of CTC in PCa patients treated with radiotherapy and characterization the CTC transcriptome signature using additional patients’ cohorts; (ii) to develop a targeting strategy toward the MIC population and validating its radiosensitizing and anti-metastatic effect, and (iii) to decipher the molecular mechanisms explaining the different roles of ALDH1A1 and ALDH1A3 in the promoting expression of the metastatic-related genes, bone colonization and remodeling, and metastasis radioresistance. Our study will identify molecular signatures that select a subpopulation of patients treated with radiotherapy that are more likely to develop bone metastases. Also, our work will help elucidate the mechanisms regulating bone MIC population and identify drugs to inhibit it, and therefore, could be of high scientific and clinical value.
- Peitzsch C, et al. Metabolic regulation of prostate cancer heterogeneity and plasticity. Semin Cancer Biol. 2022;82:94-119.
- Mukha A, et al. GLS-driven glutamine catabolism contributes to prostate cancer radiosensitivity by regulating the redox state, stemness and ATG5-mediated autophagy. Theranostics. 2021;11(16):7844-7868.
- Gorodetska I, et al. BRCA1 and EZH2 cooperate in regulation of prostate cancer stem cell phenotype. Int J Cancer. 2019;145(11):2974-2985.
- Peitzsch C, et al. Cancer stem cells: The root of tumor recurrence and metastases. Semin Cancer Biol. 2017;44:10-24.
- Krause M, et al., Cancer stem cells: Radioresistance, prediction of radiotherapy outcome and specific targets for combined treatments. Adv Drug Deliv Rev. 2017;109:63-73.
- Peitzsch C, et al. An Epigenetic Reprogramming Strategy to Resensitize Radioresistant Prostate Cancer Cells. Cancer Res. 2016;76(9):2637-51.
- Cojoc M., et al. Aldehyde Dehydrogenase Is Regulated by β-Catenin/TCF and Promotes Radioresistance in Prostate Cancer Progenitor Cells. Cancer Res. 2015;75(7):1482-94.
- Hofbauer LC. Emerging players in prostate cancer-bone niche communication. Trends Cancer 2021;7:112-121.
Molecular dissection of signaling pathways exerting bone anabolic and anti-tumor effects of physical stimuli in myeloma bone disease




→ PI Jundt: hematology, multiple myeloma, bone microenvironment, myeloma bone disease
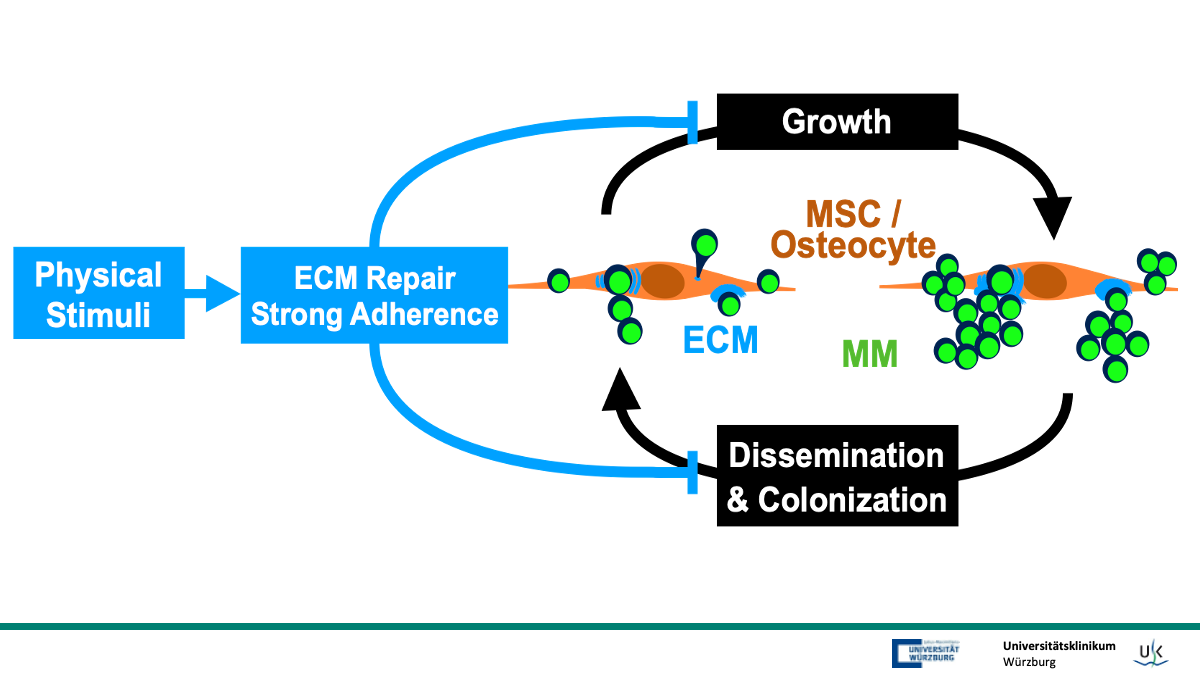
Multiple myeloma (MM) is a malignant plasma cell disorder, in which tumor cells induce osteolytic bone disease. While anabolic treatment of bone disease in malignancies is still a matter of discussion, a non-pharmacological approach to prevent or rescue osteopenia is the use of well-controlled physical stimuli. In preclinical MM studies we have shown that physical stimuli in the form of mechanical loading have promising bone-sparing outcomes and even mitigate tumor cell growth and dissemination. Transcriptome profiling of cortical osteocytes revealed substantial alterations of gene expression caused by tumor cells in a set of extracellular matrix-related genes which were rescued or stimulated by mechanical loading. Cell adhesion is an important component of mechanical loading in tissue and the tightness of adhesion mechanisms is enforced by loading. While analyzing subpopulations of MM cells in vitro that adhered with different forces to skeletal precursors we found a characteristic set of differentially expressed genes in tightly adhering MM cells. We identified a partial overlap between the in vivo and in vitro gene sets representing a convincing interactive gene network in which candidates belong to three clusters of (1) skeletal development, (2) lipid transport and (3) extracellular matrix organization. In addition, we found that low expression of lead candidates from this network is associated with worse MM patient survival using large cohorts of clinically derived RNAseq data. In this project we will molecularly dissect the networks behind adhesion / mechanotransduction and tumor homing and spread, searching for effective types of mechanical loading and innovative druggable targets that address the bone anabolic and anti-tumor effects. We hypothesize that specific lead candidates are hubs orchestrating mechanoresponsive bone remodeling and mediating rescue effects of physical stimulation in MM and bone cells. It will be proven both in vitro by interaction experiments between skeletal precursors, osteocyte cell lines and MM cells and in vivo in respective mouse models where adhesion / loading conditions are modulated using whole-body low-magnitude high frequency vibration, genetic engineering of MM cells and pharmacological intervention. This will allow for identifying targets in models of mechanical loading that can be addressed to reconstitute healthy bone tissue and at the same time impart anti-tumor effects in the microenvironment.
- Rummler M*, Ziouti F*, Bouchard AL, Brandl A, Duda GN, Bogen B, Beilhack A, Lynch ME, Jundt F*, Willie BM*, (2021) Mechanical loading prevents bone destruction and exerts anti-tumor effects in the MOPC315.BM.Luc model of myeloma bone disease. Acta Biomater., 119:247-258. *contr. equally
- Ziouti F*, Rummler M*, Steyn B, Thiele T., Seliger A., Duda G.N., Bogen, B., Willie, B.M.*, Jundt, F*, (2021) Prevention of bone destruction by mechanical loading is not enhanced by the bruton’s tyrosine kinase inhibitor CC-292 in myeloma bone disease. Int. J. Mol. Sci., 22:3840. *contr. equally
- Ziouti F*, Ebert R*, Rummler M, Krug M, Müller-Deubert S, Lüdemann M, Jakob F, Willie BM, Jundt F, (2019) Notch signaling is activated through mechanical strain in human bone marrow-derived mesenchymal stromal cells. Stem Cells Int., 2019:5150634. *contr. equally
- Müller-Deubert S*., Seefried L*., Krug M., Jakob F*., Ebert R*. (2017) Epidermal growth factor as a mechanosensitizer in human bone marrow stromal cells. Stem Cell Res., 24:69-76. *contr. equally
- Seefried L*., Müller-Deubert S*., Krug M., Youssef A., Schütze N., Ignatius A., Jakob F*., Ebert R*. (2017) Dissection of mechanoresponse elements in promoter sites of the mechanoresponsive CYR61 gene. Exp Cell Res., 354:103-111. *contr. equally
- Müller-Deubert S., Ege C., Krug M., Meißner-Weigl J., Rudert M., Bischof O., Jakob F.*, Ebert R.* (2020) Phosphodiesterase 10A as a mediator of osteogenic differentiation and mechanotransduction in bone marrow derived mesenchymal stromal cells. Stem Cells Int., 2020:7865484. *contr. equally
- Ziouti F, Prates Soares A, Moreno-Jiménez I, Rack A, Bogen B, Cipitria A, Zaslansky P, Jundt F, (2020) An early myeloma bone disease model in skeletally-mature mice as a platform for biomaterial characterization of the extracellular matrix. J. Oncol., 2020:3985315.
- Seefried L, Genest F, Strömsdörfer J, Engelmann B, Lapa C, Jakob F, Baumann FT, Sperlich B, Jundt F, (2020) Impact of whole-body vibration exercise on physical performance and bone turnover in patients with monoclonal gammopathy of undetermined significance. J Bone Oncol., 25:100323.
- Dotterweich J., Schlegelmilch K., Keller A., Tower RJ., Klein-Hitpass L., Linden C., Geyer B., Ebert R., Jakob F., Schütze N. (2016) Contact of myeloma cells induces a characteristic transcriptome signature in skeletal precursor cells – implications for myeloma bone disease. Bone, 93:155-166.
- Solimando AG., Brandl A., Mattenheimer K., Graf C., Ritz M., Ruckdeschel A., Stühmer T., Rudelius M., Dotterweich J., Ebert R., Frassanito MA., Rosenwald A., Vacca A., Einsele H., Jakob F., Beilhack A. (2018) Jam-A as a prognostic factor and new therapeutic target in Multiple Myeloma, Leukemia, 32:736-743.
Unraveling tumor-microenvironmental communication in nascent bone metastasis using zebrafish XX


→ Zebrafish
→ Lentivirus, molecular cloning
→ Live cell imaging
→ Quantitative image analysis
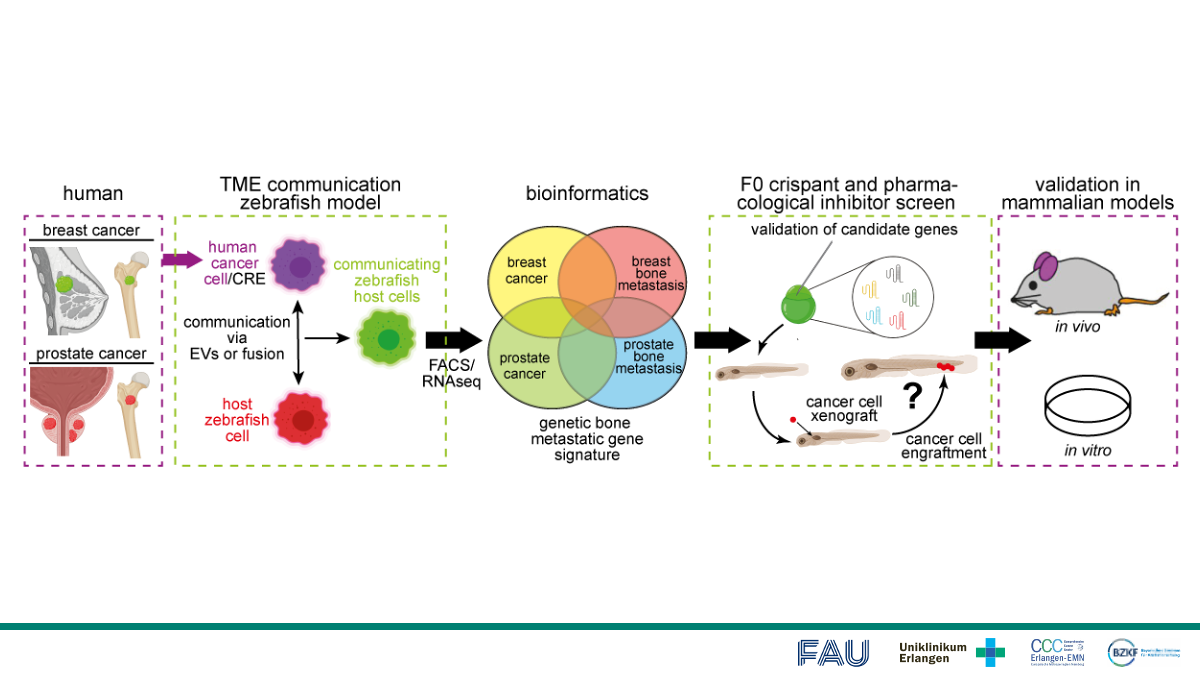
Bone metastasis contributes strongly to metastatic cancer-related death and co-morbidity. Despite its importance, mechanisms underlying bone metastasis remain poorly understood. Communication of the tumor microenvironment (TME) with the cancer cells leads to enhanced cell survival, treatment resistance, and metastasis. The in vivo study of bone metastasis is limited due to the poor accessibility of bone tissue. Therefore, we propose to utilize the zebrafish to model metastatic colonization of hematopoietic tissue by human breast and prostate cancer cells. The transparent zebrafish larva allows efficient visualization of the entire process of early metastasis by live high-resolution imaging, on a whole animal level, as well as genetic modulation of the process. To study the TME communication, we have developed a zebrafish model in which TME cells change color upon interaction with engrafted human cancer cells allowing to define interactions of circulating and disseminated tumor cells with the hematopoietic niche, the equivalent of the bone niche. Overall aim is to utilize this system to determine the shared genetic signature of the TME post-engraftment of different osteotropic cells. Additionally, we will compare the individual osteotropic cells with their maternal line to identify genes whose expression is correlated with enhanced hematopoietic niche engraftment. A bioinformatic analysis will be used to identify the most promising candidate genes as well as signaling pathways potentially controlling the TME-cancer-axis. Candidate genes and pathways will be validated by a semi-high throughput F0 crispant or pharmacological inhibitor screen. The most promising targets will be validated in vitro and in vivo mammalian systems. Finally, candidate gene expression will be assessed in human breast cancer and bone metastasis tissue. Collectively, our analysis will identify novel mechanisms of breast and prostate cancer cell colonization into the bone as well as potential markers for early diagnosis and specific targeting of bone metastases towards precision medicine.
- Groenewoud A, et al. XePhIR: the zebrafish xenograft phenotype interactive repository. Database (Oxford). 2022; 2022:baac028.
- Leone M, et al. IQGAP3, a YAP Target, Is Required for Proper Cell-Cycle Progression and Genome Stability. Mol Cancer Res. 2021; 19(10):1712-1726.
- Groenewoud A, et al. Ortho- and Ectopic Zebrafish Xeno-Engraftment of Ocular Melanoma to Recapitulate Primary Tumor and Experimental Metastasis Development. J Vis Exp. 2021; (175).
- . Emerging players in prostate cancer-bone niche communication. Trends Cancer 2021;7:112-121.
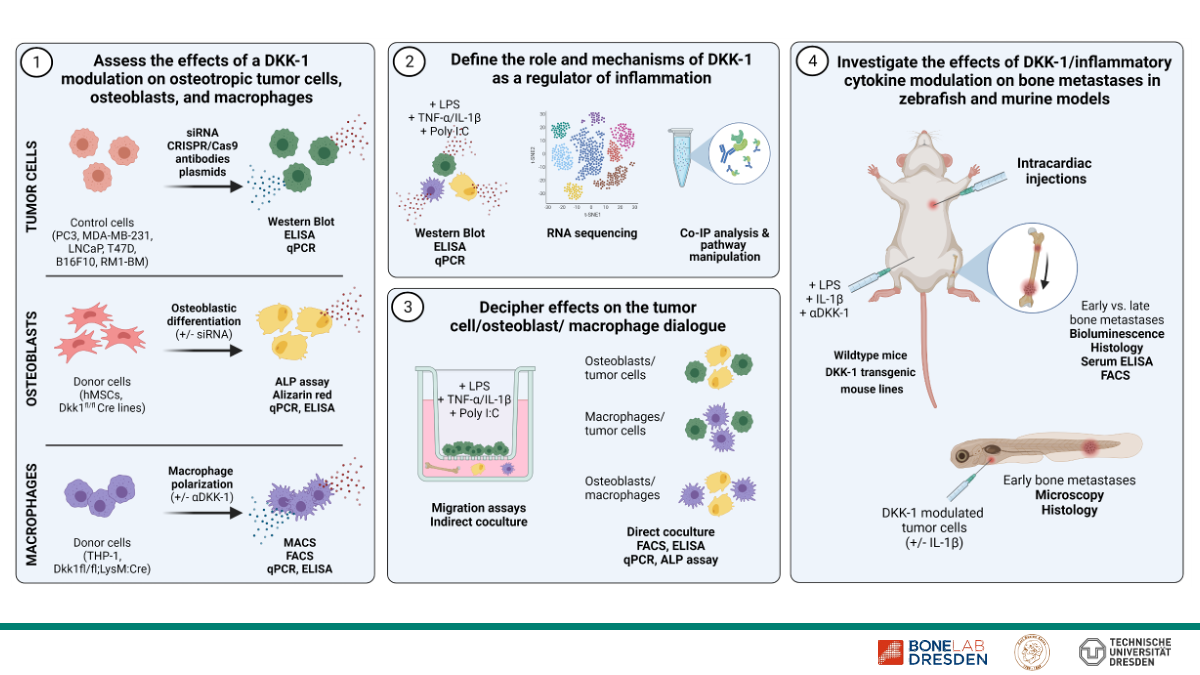
Role of Dickkopf-1 as a mediator of inflammation in osteotropic cancers


→ Intracardiac injections
→ Tumor cell lines
Dickkopf-1 (DKK-1) is an inhibitor of Wnt-signaling and best known as a key regulator of osteoblastic differentiation. In osteotropic malignancies, such as breast and prostate cancer, DKK-1 levels are increased, associated with the occurrence of bone metastases and, in some cases, linked to a poor prognosis. In recent years, DKK-1 has attained increasing attention as an important factor in tumor biology, which, in addition to its well-established effects on osteoblasts, may also mediate Wnt-independent mechanisms and interact with distinct signaling pathways.
In the first funding period of µBone, we observed that a genetic knockdown of DKK-1 but not extracellular neutralization leads to a suppression of proinflammatory cytokines such as IL-1β, CXCL8, and CXCL1 as well as inhibited migration of the osteotropic prostate cancer cell line PC3. Consistently, subcellular fractioning of proteins revealed a predominant presence of DKK-1 in the nucleus and associated membranes. Given the key importance of inflammation in bone metastases, these data point towards the identification of a novel link between DKK-1 and inflammation as a new potential therapeutic target.
Our project for the second µBone funding period aims at identifying the contribution of DKK-1 as a mediator of a proinflammatory response to early and late stages of bone metastases and if and how it sustains the dialogue between local cell types within the tumor microenvironment such as tumor cells, osteoblasts, and myeloid cells required for metastatic development.
To address this question we will apply a comprehensive panel of methods comprising genetic and pharmaceutical modulation of DKK-1 and inflammatory mediators in cell lines and primary cells to assess their bidirectional interaction. In addition, we will perform direct and indirect co-culture and migration assays upon varying treatments and combinations of the cell types. Zebrafish and mouse models including transgenic mouse lines with global and cell type-specific DKK-1 knockouts in osteoblasts and myeloid cells represent an additional central aspect of our investigations. Here, an extensive number of readouts including live imaging, FACS, ELISA, and histology will uncover different aspects of local and systemic mechanisms. Finally, clinical samples from patients with osteotropic malignancies will be used to assess local and systemic expression of DKK-1 and to investigate potential correlations.
These comprehensive analyzes will potentially reveal novel regulatory interactions between the immune system and DKK-1 and will therefore contribute to a detailed understanding if DKK-1 is suitable a therapeutic target in cancer metastasis to bone.
- Göbel A, et al. The Role of Inflammation in Breast and Prostate Cancer Metastasis to Bone. Int J Mol Sci. 2021 May 11;22:5078
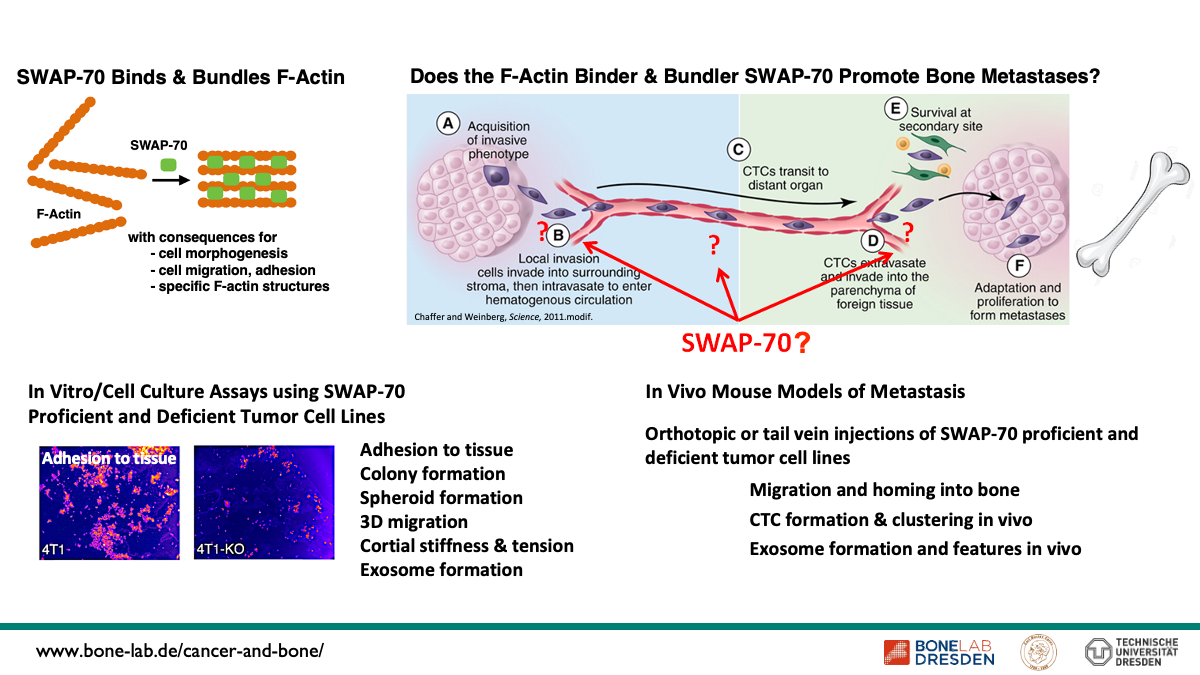
Control of Tumor Cell-Bone Metastasis by Regulation of F-actin Dynamics



→ Mouse models of tumorgenesis and metastasis
→ Biophysical assays
→ Wide range of cell biology assays related to cell migration, adhesion, cytoskeletal dynamics and related issues
→ Tumor cell lines
We propose that the control of F-actin cytoskeletal dynamics is of central importance for bone metastasis and tumor cell-bone interactions. We focus on the protein SWAP-70 which binds and bundles F-actin and was shown by us earlier to govern cell morphpogenesis, cell migration and cell adhesion as well as formation of specialized F-actin structures such as F-actin rings of osteoclasts. We further propose that SWAP-70 regulates the growth of bone metastases through its regulation of osteoclast development. Our overall hypothesis is that SWAP-70 controlled F-actin dynamics contributes to bone metastasis, and we aim at understanding the respective processes.
Our aims are:
(1) Determine the role of SWAP-70 in tumor cell bone metastasis in vivo
(2) Define SWAP-70 dependent, F-actin related features of bone metastatic tumor cells in vitro
(3) Determine how wild type bone metastases grow in SWAP-70 deficient bone
- Dohnke S, Moehser S, Surnov A, Kurth T, Jessberger R, Kretschmer K, Garbe AI. Role of Dynamic Actin Cytoskeleton Remodeling in Foxp3 + Regulatory T Cell Development and Function: Implications for Osteoclastogenesis. Front Immunol. 2022 Mar 11;13:836646.
- Betaneli V, Jessberger R. Mechanism of control of F-actin cortex architecture by SWAP-70. J Cell Sci. 2020 Jan 23;133(2):jcs233064
- Roscher A, Hasegawa T, Dohnke S, Ocaña-Morgner C, Amizuka N, Jessberger R, Garbe AI. The F-actin modulator SWAP-70 controls podosome patterning in osteoclasts. Bone Rep. 2016 Jul 19;5:214-221
- Chacón-Martínez CA, Kiessling N, Winterhoff M, Faix J, Müller-Reichert T, Jessberger R.The switch-associated protein 70 (SWAP-70) bundles actin filaments and contributes to the regulation of F-actin dynamics. J Biol Chem. 2013 Oct 4;288(40):28687-703
- Garbe AI, Roscher A, Schüler C, Lutter AH, Glösmann M, Bernhardt R, Chopin M, Hempel U, Hofbauer LC, Rammelt S, Egerbacher M, Erben RG, Jessberger R. Regulation of bone mass and osteoclast function depend on the F-actin modulator SWAP-70. J Bone Miner Res. 2012 Oct;27(10):2085-96
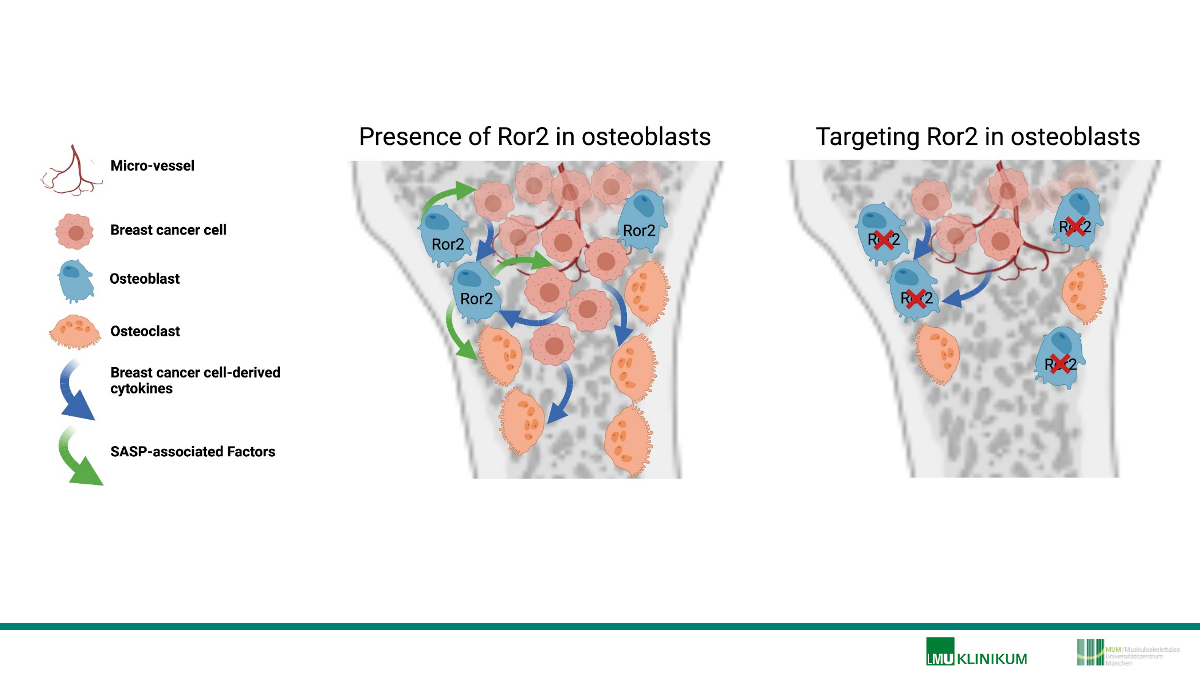
Attenuating bone metastases by inhibiting Ror2 signaling and osteoblast senescence XX




→ Bone metastasis mouse models
→ Bone histology and histomorphometry
→ RNA Therapeutics
Bone metastases are a frequent late-stage event in patients with advanced breast cancer. In the bone microenvironment, breast cancer cells disturb physiological bone remodeling by stimulating osteoclast-mediated bone resorption and suppressing osteoblast-mediated bone formation, leading to an osteolytic disease. Although anti-resorptive treatments are efficient to attenuate bone destruction, osteolytic lesions remain with the disease being incurable. Recent studies by our group and others demonstrate that affecting bone remodeling in a therapeutic manner reduces breast cancer homing to bone and metastatic growth in the bone microenvironment. Furthermore, pharmacological augmentation of bone formation protects from breast cancer-induced bone destruction. To continue this novel and innovative line of research during the second funding period of this priority program, we aim to identify unprecedented bone anabolic cues to alleviate bone metastatic burden, thereby reducing pathological bone destruction. In this context, we made the interesting observation that non-canonical Wnt signaling, inhibits bone formation. Using a comprehensive set of in vivo and in vitro approaches we uncovered that receptor of tyrosine kinase-like orphan receptor 2 (Ror2) diminishes osteoblast differentiation and bone formation. Mechanistically, Ror2-deficiency in osteoblasts impairs interleukin 6 (IL-6) signaling, which is a negative regulator of osteoblast differentiation, a key cytokine mediating the pathological cellular crosstalk in bone metastases and an important component of the senescence-associated secretory phenotype (SASP). Interestingly, while breast cancer stimulates osteoblasts to adopt a SASP, Ror2-deficient osteoblasts are resistant to develop an oxidative stress-induced SASP. These findings let us to hypothesize that inhibition of Ror2 signaling and consequently osteoblast senescence might attenuate breast cancer-induced bone destruction. To address this hypothesis, we propose to i) determine the role of Ror2 in osteoblasts in breast cancer bone metastases, ii) investigate breast cancer cell-induced Ror2-mediated osteoblast senescence, and iii) reduce Ror2 signaling and senescence to alleviate metastatic bone disease. Experimentally, we will use novel oligonucleotide-based gene silencing approaches combined with mouse genetics, metastases models and the latest technology of bone imaging and quality testing. Mechanistic insights will be elucidated using cell- and molecular biology approaches and clinical relevance of the findings will be ensured by implementing patient samples in our investigations. This project is original and expected to expand the knowledge on non-canonical Wnt signaling in bone pathology and may reveal novel targets for the development of potential future interventions to treat patients with breast cancer-induced bone disease.
- Taipaleenmäki H., Saito H. Schröder S., Maeda M., Mettler R., Ring M., Rollmann E., Gasser A., Haasper C., Gehrke T., Weiss A., Grimm S.K., Hesse E. Antagonizing microRNA-19a/b augments PTH anabolic action and restores bone mass in osteoporosis in mice. EMBO Mol Med. In Press
- Haider M.T., Ridlmaier N., Smit D.J., Taipaleenmäki H. Interleukins as Mediators of the Tumor Cell-Bone Cell Crosstalk during the Initiation of Breast Cancer Bone Metastasis. Int J Mol Sci. 22(6):2898, 2021.
- Haider M.T., Saito H., Zarrer J., Uzhunnumpuram K., Nagarajan S., Kari V., Horn-Glander M., Werner S., Hesse E., Taipaleenmäki H. Breast cancer bone metastases are attenuated in a Tgif1-deficient bone microenvironment. Breast Cancer Res. 22(1):34, 2020.
- Zarrer J., Haider M.T., Smit D.J., Taipaleenmäki H. Pathological Crosstalk between Metastatic Breast Cancer Cells and the Bone Microenvironment. Biomolecules, 10(2):337, 2020.
- Hesse E., Schröder S., Brandt D., Pamperin J., Saito H., Taipaleenmäki H. Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight, 4(9):e125543, 2019.
- Saito H., Gasser A., Bolamperti S., Maeda M., Matthies L., Jähn K., Long C.L., Schlüter H., Kwiatkowski M., Saini V., Pajevic P.D., Bellido T., van Wijnen A.J., Mohammad K.S., Guise T.A., Taipaleenmäki H.*, Hesse E*. TG-interacting factor 1 (Tgif1)-deficiency attenuates bone remodeling and blunts the anabolic response to parathyroid hormone. Nat Commun., 10(1):1354, 2019.
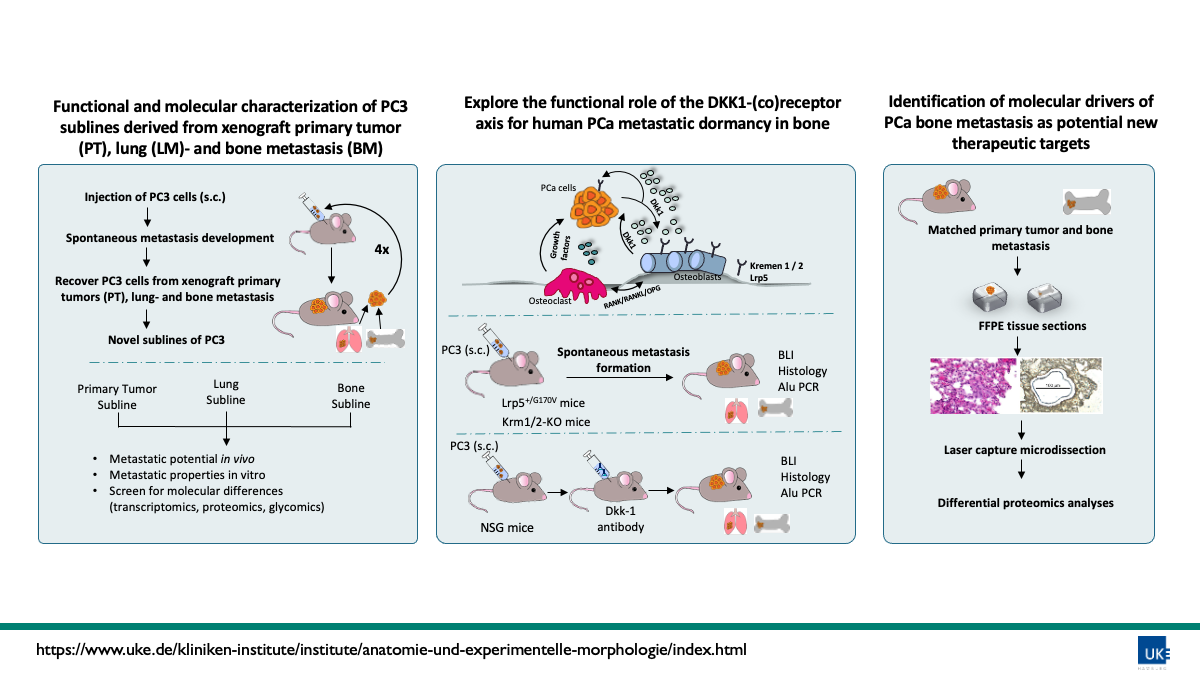
Mechanisms of metastatic outgrowth of spontaneous bone marrow metastases of human prostate cancer cells in vivo


→ Animal models
→ Tumor cell lines
→ Bone microenvironment
The functional drivers of prostate cancer (PCa) bone metastasis (BM) formation are not known yet, partly due to a lack of animal models that recapitulate the full metastatic cascade. We optimized a beyond state-of-the-art xenograft model representing the multi-step cascade of BM of human PCa. After subcutaneous (s.c.) injection of 1×106 human PCa cells (PC3 luc+, RGB+) followed by surgical resection of the primary tumor (PT, ~1cm3) these cells spontaneously spread systemically over time, mainly to the lungs and in a small percentage also to the bone. We were able to recover tumor cells from xenograft PTs, lung metastases and BM, re-injected and expanded them multiple times and ultimately established novel in vitro sublines of PC3 cells. In our experiments the incidence of single, disseminated tumor cells (DTCs) in the PC3 model is approximately 40%. In contrast, the incidence of multicellular established bone metastases is just 15% suggesting that our model essentially results in dormant DTCs in the bone. PC3 cells are known to secrete high levels of dickkopf-related protein 1 (DKK1). DKK1 regulates bone remodeling and has already been identified as regulator of metastatic latency in other tumor entities. DKK1 acts as an inhibitor of the canonical WNT signaling pathway by binding to its main receptor LRP5 or its co-receptors Kremen-1 and -2 (Krm1 and Krm2).
We aim to :
1) Analyze the functional and molecular properties of xenograft PT-, lung metastasis- and BM-derived sublines of PC3 in detail including divergent metastatic potential in vivo, metastatic properties of the cell lines in vitro, molecular differences (transcriptomics, proteomics, glycomics).
2) Explore and translate our findings regarding the functional role of the DKK1-(co)receptor axis for human PCa metastatic dormancy in bone by performing spontaneous BM xenograft experiments in Krm1-/- , Krm2-/- and Krm1/2-/- as well as Lrp5+/G170V vs. WT-NSG mice. After analyzing the role of DKK1 receptors, we will investigate the effects of targeting DKK1 itself in a translational approach by applying a clinical phase 1-2 humanized monoclonal antibody against DKK1. Additionally co-culture experiments of PC3 cells and osteoblasts / osteoclasts (obl/ocl) isolated from Krm1/2 will be performed to assess differentiated obl/ ocl counts, mRNA expression levels of obl and/ or ocl differentiation genes as well as obl and/ or ocl activity assays.
3) Identify further molecular drivers of PCa BM formation as potential new therapeutic targets using xenograft PTs and matched spontaneous BM by conducting laser capture microdissection followed by differential proteomics analyses.
In summary this project will explore the functional mechanisms of PCa bone metastasis establishment with a particular focus on the Dkk1 signaling axis.
- Labitzky V, Baranowsky A, Maar H, Hanika S, Starzonek S, Ahlers AK, Stübke K, Koziolek EJ, Heine M, Schäfer P, Windhorst S, Jücker M, Riecken K, Amling M, Schinke T, Schumacher U, Valentiner U, Lange T. Modeling spontaneous bone metastasis formation of solid human tumor xenografts in mice. Cancers (Basel) 2020;12(2):385. 7.
- Lange T, Oh-Hohenhorst SJ, Joosse SA, Pantel K, Hahn O, Gosau T, Dyshlovoy SA, Wellbrock J, Feldhaus S, Maar H, Gehrcke R, Kluth M, Simon R, Schlomm T, Huland H, Schumacher U. Development and characterization of a spontaneously metastatic patient-derived xenograft model of human prostate cancer. Sci Rep 2018;8(1):17535.
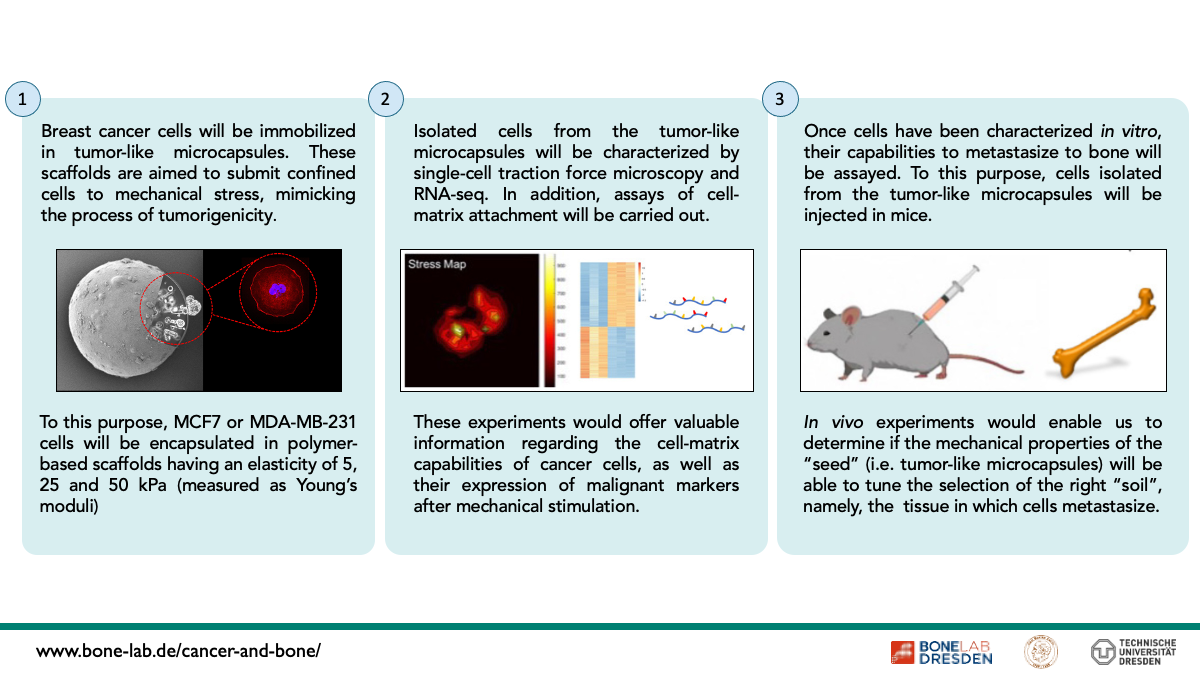
Bone osteotropism: Influence of confinement and mechanical properties of the seeds in the selection of the soil


→ Design and performance of polymer-based tumor-like scaffolds.
→ Cell encapsulation.
→ Single-cell traction force microscopy.
One of the most slippery questions in cancer pathologies, is why metastatic cells target several organs instead of others. A very interesting example is observed in case of breast or prostate cancers, which are likely metastasizing into bone, instead of other organs, in a phenomenon known as osteotropism.
Thus, this project intends to address this question from an interdisciplinary perspective, searching for a link between the biomechanical stress sensed by confined cells within primary tumours, and the ability of these cells to invade, colonize and form secondary tumours into bone tissues.
To this purpose, MCF7 and MDA-MB-231 breast cancer cell lines will be entrapped and cultured in artificial primary tumours having elasticities of 5.0, 25 and 50 kPa (measured as Young’s Moduli), before being characterized at biophysical level by single cell traction force microscopy and cell adhesiveness onto artificial bone-like scaffolds. These experiments will be enriched by sampling cells at different time-points post-cell immobilization, with the purpose to create a mechano-temporal map of cancer biomechanics in vitro.
Results obtained at biomechanical level will be then supported by RNA-array studies, enabling us finding a physic-molecular correlation between mechanical stress and expression of markers involved in bone metastasis.
In the final step of this project, in vitro assays carried out at biomechanical and genetic level will be validated by in vivo studies. Thus, cell invasion assays and formation of secondary tumours will be assessed in mice models, after pre-conditioning cancer cells to the experimental milieus described above.
In vitro and in vivo results acquired with breast cancer cells will be compared to those obtained with the glioma cell line U87-MG, which has been described to have low metastatic affinity for bone, being used as biological control.
According to our knowledge this proposal represents the first clear and systematic attempt to understand the influence of the mechanical properties of the seed in the selection of the soil, unravelling relevant knowledge in the field of mechanics of cell invasion, which can be further used to develop new treatments against malignant diseases.
- Ertekin, et al. 3D hydrogel-based microcapsules as an in vitro model to study tumorigenicity, cell migration and drug resistance. Acta Biomaterialia. 2022. April 1; 142: 208.
- Fuentes-Chandía, et al. 3D Spheroids Versus 3D Tumor-Like Microcapsules: Confinement and Mechanical Stress May Lead to the Expression of Malignant Responses in Cancer Cells. Advanced Biology. 2021. May 7. 5: 2000349
- Leal-Egaña et al. Re-engineering Artificial Neoplastic Milieus: Taking Lessons from Mechano- and Topobiology. Trends in Biotechnology. 2020. February 1. 38: 142.
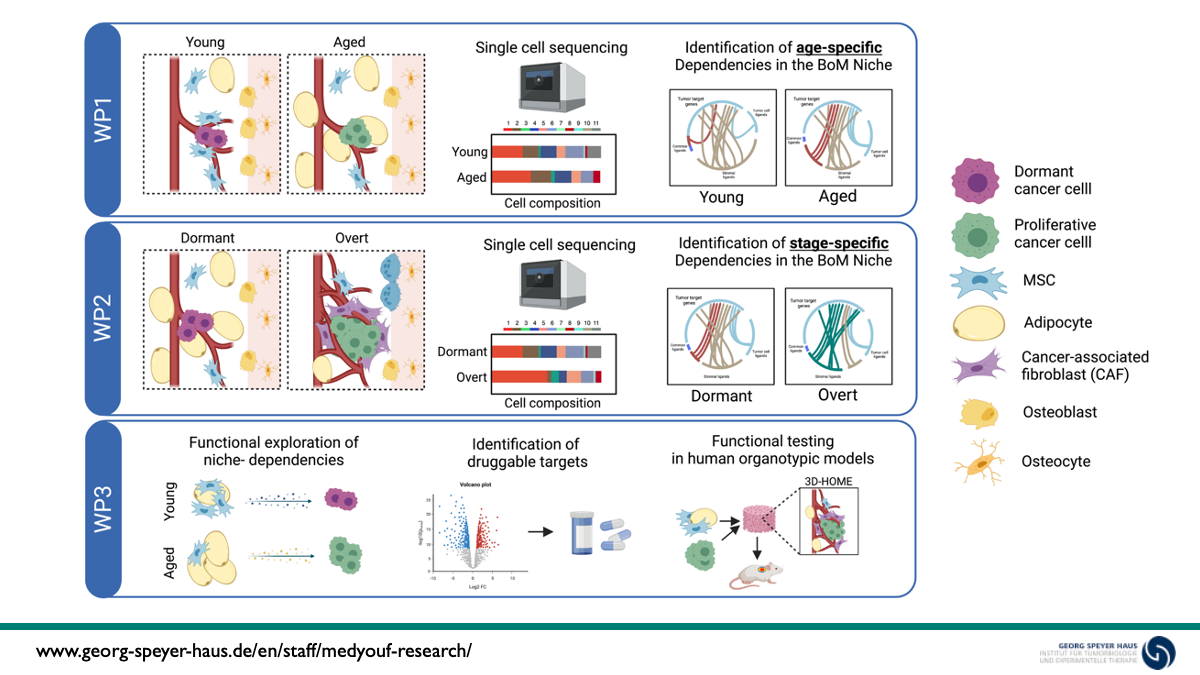
Dissecting the Role of ECM-producing Stromal Cells in Bone Metastatic Progression cancers XX


→ 3D Human Organotypic Marrow Environments (3D-HOMEs)
→ 3-dimensional whole-bone imaging at single cell resolution
→ Single cell transcriptomics including 10x workflow and data analysis
Despite substantial advances in the treatment of primary tumors, disseminated disease remains the major source of cancer-related deaths. In breast cancer, bone is one of the major sites of metastasis and the clinical relevance of disseminated tumor cells (DTCs) is highlighted by the fact that their detection at the time of diagnosis correlates with poor outcome. While cues leading to the activation of dormant DTCs remain poorly understood, advanced age is one of the most significant predictors of overt bone metastasis occurrence.
In the first funding period, we report that alterations in the bone marrow niche, which are reminiscent of physiological aging, including cellular architecture, extracellular matrix composition and bone quality, dictate growth dynamics of breast cancer DTCs in bone. These observations point to a prominent tumor-extrinsic control of dormancy that is linked to aging of structural niche cell types. To confirm these data in a human setting and dissect the cellular origin of the observed phenotype, we used bone metastatic cell lines and patient-derived circulating tumor cells (CTCs) in a newly established 3D human organotypic marrow environment (3D-HOME) system, which enabled us to pinpoint the cellular-drivers of the phenotype and demonstrate that age-related alterations in the niche, indeed drive the awakening of quiescent metastatic cancer cells.
Within the second funding period we will take a holistic approach to dissect the molecular mechanisms that underlie these dependencies and explore the dynamically evolving niche landscape in bone metastasis in a temporally and spatially resolved fashion, in order to understand the mechanisms by which tumor/ niche interaction shapes the metastatic environment and define common versus disease-stage specific therapeutic targets. This work program is articulated in 3 work packages that aim to (i) dissecting the the reciprocal crosstalk between tumor and niche cells at early stages post-colonization, in young vs aged niches, (ii) explore the dynamic switch in proximal niche composition and priming in early versus late stage metastasis to unravel evolving tumor vulnerabilities and (iii) explore the metabolic dependencies of tumor cells in normal vs aged environment to identify potentially druggable metabolic dependencies at early disease stage.
- Tirado-Gonzalez I, Czlonka E, Nevmerzhitskaya A, Soetopo D, Bergonzani E, Mahmoud A, Contreras A, Jeremias I, Platzbecker U, Bourquin JP, Kloz U, Van der Hoeven F, Medyouf H. CRISPR/Cas9-edited NSG mice as PDX models of human leukemia to address the role of niche-derived SPARC. Leukemia. 2018 doi: 10.1038/leu.2017.346.
- Medyouf H The microenvironment in human myeloid malignancies: emerging concepts and therapeutic implications. Blood. 2017;129(12):1617-1626. Invited Review Series.
- Medyouf H*, Mossner M, Jann JC, Nolte F, Raffel S, Herrmann C, Lier A, Eisen C, Nowak V, Zens B, Müdder K, Klein C, Obländer J, Fey S, Vogler J, Fabarius A, Riedl E, Roehl H, Kohlmann A, Staller M, Haferlach C, Müller N, John T, Platzbecker U, Metzgeroth G, Hofmann WK, Trumpp A*, Nowak D . Myelodysplastic cells in patients reprogram mesenchymal stromal cells to establish a transplantable stem cell niche disease unit. Cell Stem Cell, 2014. 824-837. * Co-corresponding authors
- Ehninger A, Boch T, Medyouf H, Müdder K, Orend G, Trumpp A. Loss of SPARC protects hematopoietic stem cells from chemotherapy toxicity by accelerating their return to quiescence. Blood. 2014. 123(26):4054-63.
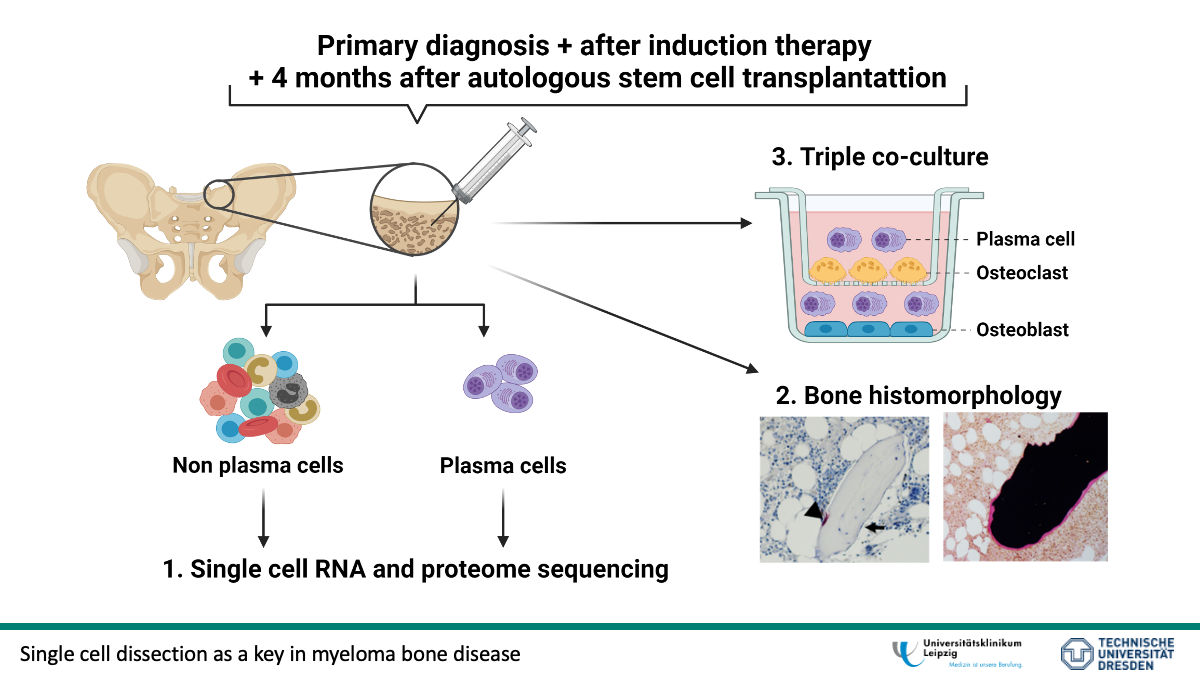
Deciphering pathogenesis, treatment and reversibility of myeloma bone disease at a single cell resolution


Multiple myeloma (MM) is a monoclonal plasma cell disease of the bone marrow in which up to 80% of newly diagnosed patients have osteolytic lesions. In the last years, treatment options for patients with MM have improved by providing anti-resorptive treatment in addition to conventional therapy to reduce the activity of bone-resorbing osteoclasts. However, subsequent improvement in bone phenotype rarely occurs despite achieving deep remission and the patients suffer from an impaired quality of life. Why MM bone lesions do not often show recalcification has not been sufficiently studied. Therefore, the proposed project will investigate the pathomechanism of bone destruction before and after therapy using longitudinal samples. We postulate that the failure to calcify is due to impaired bone formation. Fibroblast growth factor-23 (FGF-23) may play an important role, as plasma levels of FGF-23 are increased in MM. To test our hypothesis, bone marrow aspirates, bone biopsies, and plasma samples will be collected at primary diagnosis as well as before and after autologous stem cell transplantation. Longitudinal changes in the malignant plasma cell compartment and surrounding bone marrow microenvironment from patients with MM will be studied by single cell RNA and surface proteome sequencing. To identify crucial factors that are responsible for the lack of bone healing, we will compare patients with and without recalcification of osteolytic lesions after therapy. The extent of bone disease will be assessed using bone mineral density measurements and computed tomography. Bone morphology will be characterized further with histology, analyzing not only osteoclasts and osteoblasts but also bone mineralization, which has an influence on bone formation. The obtained data will be correlated with blood parameters (hemoglobin, red blood cells) and bone-specific plasma parameters (e.g. FGF-23, OCN, CTX), again longitudinal changes in patients with MM are important. In order to investigate the influence of autocrine/paracrine factors and therapy on osteoblast function as well as the potential for recalcification, we will develop an in vitro triple co-culture of osteoblasts, osteoclasts and plasma cells. Furthermore, the model will be used to validate findings from single cell experiments and to study the differentiation potential of patient-derived mesenchymal stem cells before and after therapy as well as the influence of induction therapy on healthy cells. Besides myeloma-specific therapeutic interventions, we will evaluate the blockage of FGF23. If single cell sequencing identifies candidates associated with impaired osteoblast function, their expression will be regulated in triple co-culture. Within the current study, factors responsible for the failure of bone regeneration after successful therapy of newly diagnosed MM will be identified. The long-term goal is to develop a therapeutic intervention for the alleviation of bone disease in MM.
- Spatiotemporal assessment of immunogenomic heterogeneity in multiple myeloma. Merz M, Hu Q, Merz AMA, Wang J, Hutson N, Rondeau C, Celotto K, Belal A, Alberico R, Block A, Mohammadpour H, Wallace PK, Tario JD, Luce J, Glenn ST, Singh PK, Samur MK, Munshi NC, Liu S, McCarthy PL, Wei L, Hillengass J. Blood Adv. 2022 Jul 22:bloodadvances.2022007457.
- Deciphering spatial genomic heterogeneity at a single cell resolution in multiple myeloma. Merz M, Merz AMA, Wang J, Wei L, Hu Q, Hutson N, Rondeau C, Celotto K, Belal A, Alberico R, Block AW, Mohammadpour H, Wallace PK, Tario J, Luce J, Glenn ST, Singh P, Herr MM, Hahn T, Samur M, Munshi N, Liu S, McCarthy PL, Hillengass J. Nat Commun. 2022 Feb 10;13(1):807.
- Predictive value of longitudinal whole-body magnetic resonance imaging in patients with smoldering multiple myeloma. Merz M, Hielscher T, Wagner B, Sauer S, Shah S, Raab MS, Jauch A, Neben K, Hose D, Egerer G, Weber MA, Delorme S, Goldschmidt H, Hillengass J. Leukemia. 2014 Sep;28(9):1902-8.
- Luspatercept mitigates bone loss driven by myelodysplastic neoplasms and estrogen-deficiency in mice. Weidner H, Wobus M, Hofbauer LC, Rauner M, Platzbecker U. Leukemia. 2022. in press.
- Interactions of Anemia, FGF-23, and Bone in Healthy Adults-Results From the Study of Health in Pomerania (SHIP). Hannemann A, Nauck M, Völzke H, Weidner H, Platzbecker U, Hofbauer LC, Rauner M, Baschant U. J Clin Endocrinol Metab. 2021 Jan 1;106(1):e288-e299.
- Increased FGF-23 levels are linked to ineffective erythropoiesis and impaired bone mineralization in myelodysplastic syndromes. Weidner H, Baschant U, Lademann F, Ledesma Colunga MG, Balaian E, Hofbauer C, Misof BM, Roschger P, Blouin S, Richards WG, Platzbecker U, Hofbauer LC, Rauner M. JCI Insight. 2020 Aug 6;5(15):e137062.
The role of stromal cells in cancer cell homing and growth XX




→ in vivo cancer models
→ Cell and molecular biology
We are particularly interested in understanding the role of the supporting stromal cells in the development of bone metastasis and cancer progression in patients with breast and prostate cancer.
Breast and prostate cancer cells can home to the bone marrow, where they presumably hijack the hematopoietic stem cell niche. They may stay dormant, but for unknown reasons sometimes start to proliferate giving rise to bone metastatic lesions. We have shown that decreasing the number of stromal cells pharmacologically enhances cancer cell homing to the bone marrow in mice. Initial characterization confirmed a relationship with two mesenchymal subpopulations, one that produces fibronectin and one that probably responds to it. We would like to define these two subpopulations further using transgenic mouse models. This will be followed by pharmacologic manipulation in mice in order to increase the identified subpopulation(s) and diminish cancer cell homing and consequently bone metastatic disease. The relevance of this population will be evaluated in bone marrow samples obtained from patients with breast and prostate cancer, and correlated with the presence of tumor cells in the bone marrow and prognosis of the disease. The ultimate aim of this part is to manipulate the stromal cells to make the premetastatic niche hostile to cancer cell, and possibly to eject them from the niche entirely.
Furthermore, the bone marrow stromal cells can suppress tumor growth when injected together with tumor cells. We identified a subpopulation of stromal cells that (in contrast to other subpopulations) is able to inhibit tumor progression on its own. Here too we found another subpopulation representing 1% of the bone marrow nucleated cells that in the absence of fibronectin can no longer suppress growth. We will take advantage of transgenic models to better characterize these two subpopulations. Interestingly, an immune cell population was increased every time cancer growth was suppressed. We therefore aim to understand how the immune response is modulated in the presence of the stromal cell subpopulations. Based on our findings, we plan an evaluation of tumor sections to correlate the expression of markers that define the inhibitory stromal population with the prognosis of the tumor in patients with both breast and prostate cancer. Understanding the mechanism that underlies the suppression of cancer growth by stromal cells might open novel avenues in cancer therapy.
- H. Ghura, M. Keimer, A. von Au, N. Hackl, V. Klemis, I.A. Nakchbandi “Inhibition of fibronectin accumulation suppresses tumor growth” Neoplasia. 2021 September;23(9):837-850.
- F. Wirth, K. Huck, A. Lubosch, C. Zoeller, S. Porubsky, I.A. Nakchbandi. “Cdc42 in osterix-expressing cells alters osteoblast behavior and myeloid lineage commitment”. Bone. 2021 Dec;153:116150. doi: 10.1016/j.bone.2021.116150. Epub 2021 Aug 13.
- S. Rossnagl, H. Ghura, C. Groth, E. Altrock, F. Jakob, S. Schott, P. Wimberger, T. Link, J.-D. Kuhlmann, A. Stenzl, J. Hennenlotter, T. Todenhöfer, M. Rojewski, K. Bieback, I.A. Nakchbandi. “A Subpopulation of Stromal Cells Controls Cancer Cell Homing to The Bone Marrow”. Cancer Res. 2018 Jan 1;78(1):129-142.
- S. Rossnagl, E. Altrock, C. Sens, S. Kraft, K. Rau, M.D. Milsom, T. Giese, Y. Samstag, I.A. Nakchbandi. “EDA-Fibronectin Originating from Osteoblasts Inhibits the Immune Response against Cancer”. 2016; PLoS Biol 14(9):e1002562.
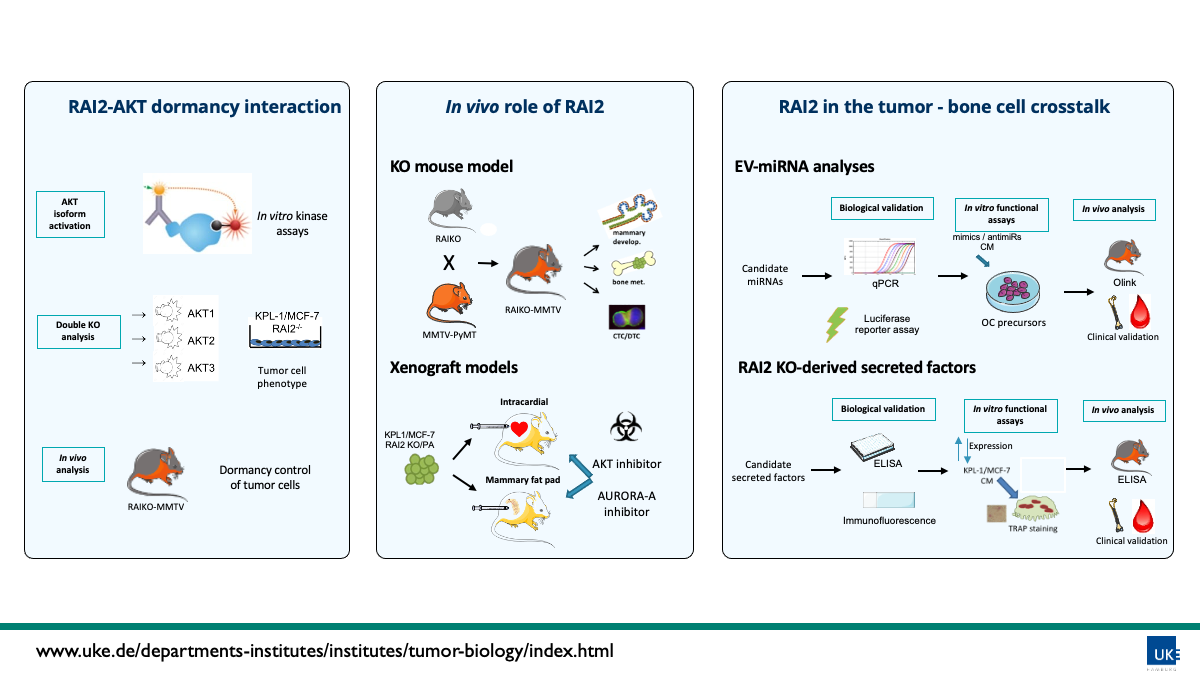
RAI2 as a new inhibitor of the dissemination of cancer cells in the bone microenviroment



→ DTCs and CTCs analysis
→ Mouse models
Bone marrow (BM) is a common homing organ for disseminated tumor cells (DTCs, the seed of metastases) derived from various types of malignant epithelial tumors. We have shown that the presence of single DTCs in the BM is an independent predictor of metastatic relapse in many different solid tumors including breast cancer and identified genes, expression signatures and mechanisms associated with an early tumor cell dissemination to the BM. We identified retinoic acid–induced 2 (RAI2) as a novel metastasis suppressor gene significantly associated with both positive DTC status and poor prognosis in breast cancer. Furthermore, our functional studies showed that RAI2 sustains differentiation of ER-positive tumor cells, whereas loss of RAI2 induces hormone independent growth and activation of AKT signaling as an important mediator of dormancy control. Xenograft experiments performed during the first µBONE funding period showed an increased tumor dissemination (increased numbers of CTCs and DTCs) when RAI2 KO cells where injected orthotopically in Scid mice. Therefore, our results indicate that loss of RAI2 expression might represent a so far undiscovered key event for the onset of metastatic progression in the bone. The primary goal of this project is thus to better understand the precise role of RAI2 for the development of bone metastasis in breast cancer. Our recent drug screenings, identified a possible synthetic lethality associated with RAI2 expression in combination with drugs such as Aurora-A and AKT inhibitors. Thus, the role of RAI2 dependent AKT regulation in survival and dormancy will be analyzed in this project (WP1) as well as it suitability as a drug target in mouse models (WP 2.2). Also, the possibility of using Aurora-A kinase inhibition in this context will be analyzed in WP2.2. We have also established a RAI2 KO (RAIKO) mouse, showing that RAI2 does not appear to be involved in initial tumor development. In this project, we will cross RAIKO with a mouse model with spontaneous breast tumor formation (MMTV) to further analyze the involvement of RAI2 in early tumor dissemination and bone metastasis (WP2.1). Finally, we found that both the total conditioned media as well as the EV fraction from RAI2 KO cells was significantly increasing osteoclast differentiation. Thus, in the last WP3, we plan to analyze: i) RAI2 dependent EV-related miRNA expression in bone modulation, and ii) investigate of the role of RAI2 KO-derived secreted factors in tumor-bone crosstalk. Thus, the results obtained in the ongoing and future grant will contribute to a better understanding of cancer dormancy and metastatic development in the BM microenvironment.
- Werner S, … et. al, Pantel K, Wikman H. Suppression of early hematogenous dissemination of human breast cancer cells to bone marrow by retinoic Acid-induced 2. Cancer Discov. 2015 May;5(5):506-19.
- Pereira-Veiga T, Schneegans S, Pantel K, Wikman H. Circulating tumor cell-blood cell crosstalk: Biology and clinical relevance. Cell Rep. 2022 Aug 30;40(9):111298.
- Lang A, Goradia N, Wikman H, Werner S, Wilmanns M, Ohlenschläger O. 1H, 13C, and 15N backbone assignments of the C-terminal region of the human retinoic acid-induced protein 2. Biomol NMR Assign. 2020 Oct;14(2):271-275.
- Besler K, … et. al, Pantel K, Wikman H, Werner S. Expression Patterns and Corepressor Function of Retinoic Acid-induced 2 in Prostate Cancer. Clin Chem. 2022 Jul 3;68(7):973-983.
- Hofbauer LC, … et. al, Pantel K. Novel approaches to target the microenvironment of bone metastasis. Nat Rev Clin Oncol. 2021 Apr 19.
- Keller L, Pantel K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat Rev Cancer. 2019 Oct;19(10):553-567.
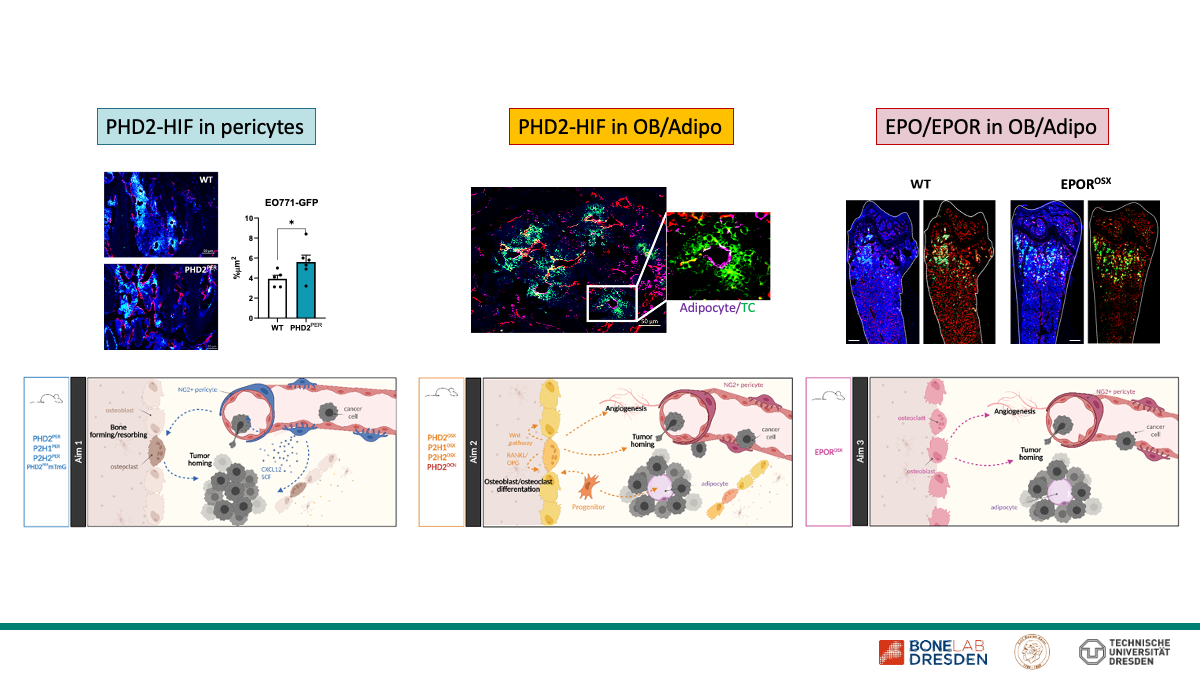
The impact of HPPs in bone marrow niche cells during metastasis homing to bone



→ Cell lines and mouse models
→ Hypoxia Animal models
→ Bone metastasis homing model with intracardiac injection
→ Detection of tumor cells in bone by immunofluorescence
→ µCT and bone histomorphometry
→ Conditional transgenic mouse lines in hypoxia signaling (HIF1-2α/PHD2-3) – ΔCre-lines
All cells in our body require sufficient oxygen pressure to function properly. Therefore, insufficient oxygen supply (hypoxia) is a prominent feature in various physiological and pathological conditions, including bone metabolism, tumor development and metastasis. The central mediators during deprived oxygen are hypoxia inducible factors (HIF), transcription factors that are tightly regulated by oxygen-dependent HIF prolyl hydroxylases (PHDs), and drivers of various biological processes. During the first funding period, we revealed that the PHD2-HIF2-VCAM1 axis in bone marrow endothelial cells is essential for homing of breast carcinoma cells to the bone, and that PHD2-HIF2-Erythopoietin (EPO)/EPOR in osteoblasts and their progenitors directly regulates bone metabolism impacting growth of metastatic tumor cells. Furthermore, in view of SPP2084, we recently expanded our unique collection of hypoxia pathway protein transgenic mouse lines to more carefully interrogate the role of pericytes and mature osteoblasts/bone marrow adipocytes, and in a set of preliminary bone metastasis experiments, we identified new phenotypes that underscore their role in tumor cell homing to bone. Within this new project proposal, we are therefore well equipped to further unravel the impact of the PHD-HIF axis and its downstream mechanisms during homing and growth of disseminated tumor cells in bone. Taken together, we are convinced that with this project we will provide further insights into the role of HPPs in the interaction between tumor cells, bone vasculature and bone cells that may provide new therapeutic strategies to interrupt the malignant crosstalk and ameliorate the formation of bone metastases.
- Epo/EpoR signaling in osteoprogenitor cells is essential for bone homeostasis and Epo-induced bone loss.
Rauner M, Murray M, Thiele S, Watts D, Neumann D, Gabet Y, Hofbauer LC, Wielockx B.
Bone Res. 2021 Sep 13;9(1):42. doi: 10.1038/s41413-021-00157-x - Hypoxia-Inducible Factors Regulate Osteoclasts in Health and Disease.
Meng X, Wielockx B, Rauner M, Bozec A.
Front Cell Dev Biol. 2021 Mar 18;9:658893. doi: 10.3389/fcell.2021.658893. - Hypoxia Pathway Proteins are Master Regulators of Erythropoiesis.
Watts D, Gaete D, Rodriguez D, Hoogewijs D, Rauner M, Sormendi S, Wielockx B.
Int J Mol Sci. 2020 Oct 30;21(21):8131. doi: 10.3390/ijms21218131